Epithelial Lesions of the Oral Cavity, Larynx, Trachea, Nasopharynx, and Paranasal Sinuses
HISTOLOGIC RECALL
As briefly described in Chapter 19, the oral cavity (including the palate, tongue, pharynx, and floor of the mouth) is lined by squamous epithelium with varying degrees of
surface keratinization. The surface of the larynx, facing the oral cavity, is also lined by squamous epithelium. The inner aspects of the larynx (including the vocal cords) are lined by a nonkeratinizing epithelium composed of five to six layers of parabasal and intermediate squamous cells. Lower aspects of the larynx and the adjacent trachea are, in part, lined by similar nonkeratinizing epithelium and, in part, by ciliated epithelium, identical to bronchial epithelium, described in Chapter 19. The paranasal sinuses and the nasopharynx are principally lined by an epithelium composed of cuboidal and columnar ciliated cells. All ciliated epithelia contain mucus-producing goblet cells and may undergo squamous metaplasia, as described in Chapter 19.
surface keratinization. The surface of the larynx, facing the oral cavity, is also lined by squamous epithelium. The inner aspects of the larynx (including the vocal cords) are lined by a nonkeratinizing epithelium composed of five to six layers of parabasal and intermediate squamous cells. Lower aspects of the larynx and the adjacent trachea are, in part, lined by similar nonkeratinizing epithelium and, in part, by ciliated epithelium, identical to bronchial epithelium, described in Chapter 19. The paranasal sinuses and the nasopharynx are principally lined by an epithelium composed of cuboidal and columnar ciliated cells. All ciliated epithelia contain mucus-producing goblet cells and may undergo squamous metaplasia, as described in Chapter 19.
Minor salivary glands are dispersed throughout the oral cavity and adjacent organs. The tumors of these glands can be sampled only by aspiration biopsy. Aspiration biopsy may also be used for the study of deeply seated tumors of the various component organs and bony structures (Castelli et al, 1993; Das et al, 1993; Gunhan et al, 1993; Mondal and Raychoudhuri, 1993; Mathew et al, 1997; Domanski and Akerman, 1998; Shah et al, 2000). These issues are discussed in Chapters 32 and 36.
ORAL CAVITY
SAMPLING TECHNIQUES
Lesions of the oral cavity can be sampled by smears obtained by scraping. In most cases, the scrape smears may be obtained with a simple tongue depressor or a small curette. For oral lesions covered with thick layers of keratin, a more vigorous scraping with a sharp metallic instrument may be advisable. A brush specifically designed to sample oral lesions was described by Sciubba (1999).
INDICATIONS FOR CYTOLOGIC EXAMINATION
The principal application of cytologic techniques to epithelial lesions of the oral cavity is the diagnosis of occult carcinomas, not identified or not suspected on clinical inspection. As will be set forth in this chapter, cytologic methods are particularly valuable in screening for occult oral cancer, but may occasionally contribute to the diagnosis of early or unsuspected cancers of adjacent organs. King (1962) briefly summarized the early history of the application of cytologic techniques to lesions of the oral cavity.
CYTOLOGY OF ORAL SQUAMOUS EPITHELIUM IN THE ABSENCE OF DISEASE
Squamous Epithelial Cells
Normal squamous epithelium of the oral cavity sheds superficial and intermediate squamous cells, identical to squamous cells of the vagina and cervix, except that nuclear pyknosis is not observed. Such cells occur either singly or in clusters and are identical with squamous cells that are found in specimens of sputum and of saliva (see Chap. 19).
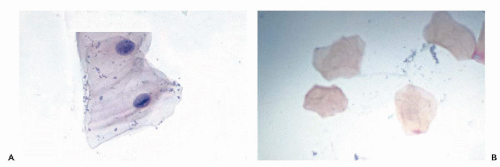
A longitudinal condensation of the nuclear chromatin in the form of a nuclear bar with lateral extensions, similar to that observed in Anitschkow cells in the myocardium in rheumatic heart disease, has been recorded in superficial squamous cells by Wood et al (1975). A similar cell change was also illustrated in the Atlas of Oral Cytology by Medak et al (1970). Such cells are commonly seen in smears of the mucosal surface of the lower lip and the adjacent floor of the mouth in perfectly healthy people (Fig. 21-1A). The change is probably related to “nuclear creases” but its significance is unknown. Similar cells may be observed in mesothelial cells in the pericardium surface of the conjunctiva and in other organs.
Fully keratinized squamous superficial cells without visible nuclei (keratinized squames) are a common component of oral smears, especially from the palate, and do not necessarily reflect a significant abnormality (Fig. 21-1B). All stages of transition between nonkeratinized and keratinized cells may be observed. Smaller parabasal squamous cells may be observed if the surface of the epithelium is vigorously scraped, or if an epithelial defect, such as an ulceration, is present.
In general, the cytology of the oral cavity in the absence of disease is simple and monotonous.
Squamous oral cells carry on their membranes blood group antigens (for review, see Dabelsteen et al, 1974).
Other Cells
Mucus-producing columnar cells originating in the nasopharynx or the salivary gland ducts may occasionally be observed. A vigorous scrape of the tonsillar area or the base of the tongue may result in shedding of lymphocytes, singly or in clusters.
Oral Flora
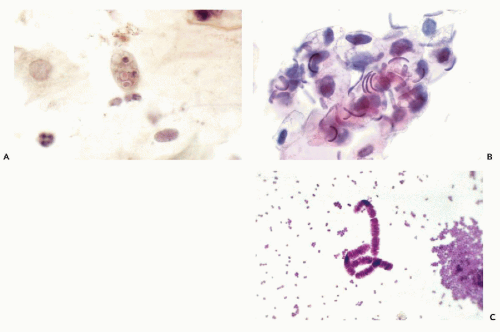
Oral flora, especially in patients with poor oral hygiene, is rich in a variety of saprophytic fungi and bacteria. A protozoon, Entamoeba gingivalis, is fairly common (Fig. 21-2A). It is a multinucleated organism larger than Amoeba histolytica, from which it differs because it does not phagocytize red blood cells (see Chaps. 10 and 24). The presence of these organisms does not necessarily indicate an inflammatory process in the oral cavity. An unusual organism, Simonsiella species, was described in smears of oropharynx, sputum, and gastric aspirates by Greenebaum et al (1988). The large bacteria form caterpillar-like chains, each composed of 10 to 12 individual bacteria. The bacterial chains are readily observed overlying squamous cells (Fig. 21-2B). The organism is nonpathogenic, most likely to be observed in mouths of people with rich dietary intake, particularly fat and proteins.
Buccal Squamous Cells in Genetic Counseling and as a Source of DNA
Buccal smears are the cheapest and easiest-to-use laboratory test to determine genetic sex, by observing and counting sex chromatin (Barr bodies) in squamous oral cells. The Barr bodies can be recognized as a half-moon shaped chromatin condensation at the nuclear membrane (see Chaps. 4, 7, 8, 11, and 29). Although, theoretically, in genetic females all squamous cells with nonpyknotic, open vesicular nuclei should contain a Barr body, in practice, it can be identified in fewer than half of these cells by light microscopy of oral smears stained with Papanicolaou’s stain. Further, peripherally placed chromocenters and focal thickening of the nuclear membrane may mimic Barr bodies. There is some controversy about whether the frequency of
visible sex chromatin varies during the menstrual cycle (Chu et al, 1969; Ashkenazi et al, 1975).
visible sex chromatin varies during the menstrual cycle (Chu et al, 1969; Ashkenazi et al, 1975).
For all practical intents and purposes, the finding of about half a dozen or more cells with a clear-cut single sex chromatin body is diagnostic of the XX female chromosomal constitution (see Chap. 4). An excess of Barr bodies (very rarely more than two in a cell) indicates an excess of X chromosomes (“superfemale,” with cells containing 47 chromosomes with XXX). Occasionally, malignant cells may contain two or more Barr bodies, reflecting aneuploidy.
The presence of Barr bodies in cells in a phenotypic male strongly suggests Klinefelter’s syndrome (47 chromosomes, YXX). The absence of Barr bodies in a phenotypic female suggests Turner’s syndrome or another form of gonadal dysgenesis (see Chap. 9).
Buccal cells collected in mouthwash or by other techniques may be valuable as a source of DNA for various tests, including person identification (Heath et al, 2001).
INFLAMMATORY DISORDERS
Acute and Chronic Inflammatory Processes
Superficial erosion or ulceration of the squamous epithelium occurs frequently in the course of diffuse or localized inflammatory processes or poor oral hygiene. As a result, the normal population of superficial and intermediate squamous cells in smears is partially or completely replaced by parabasal squamous cells from the deeper epithelial layers. Such cells may vary in size and shape; their principal feature is relatively large, occasionally multiple, round or oval vesicular nuclei of monotonous sizes. As is common in nuclei of younger cells, chromocenters may be readily observed against a pale nuclear background; occasionally, small nucleoli may be noted. The cytoplasm is often poorly preserved (Fig. 21-3). In the presence of a diffuse stomatitis or gingivitis, the preponderance of the irregularly shaped parabasal cells may result in an initial impression of a significant epithelial abnormality; close attention to nuclear detail will prevent an erroneous diagnosis of cancer.
In chronic ulcerative processes, mono- and multinucleated macrophages may also occur. Purulent exudate or leukocytes of various types are a common component of smears in these situations. Plasma cells are frequently observed, particularly in smears from the posterior oral cavity or pharynx.
Specific Inflammatory Disorders
Actinomycosis
As discussed in Chapter 19, bacteria of the Actinomyces species are common saprophytes of the oral cavity, usually found within tonsillar crypts. They may be acquired by chewing on bacterium-carrying plants, and are usually of no clinical significance. However, they may invade the traumatized or ulcerated mucosa and form an abscess or sinus tract. As discussed in Chapters 10 and 19, the organism can be recognized in Papanicolaou-stained oral smears as masses of matted bacterial filaments. The identity of the organism should be confirmed by culture if there is clinical evidence of an inflammatory process. The presence of Actinomyces in oral smears must always be correlated with clinical findings to distinguish between saprophytic and pathogenic organisms.
Oral Herpes
This common disorder, characterized by blisters and painful ulcerations, is caused by Herpesvirus type 1 that can be identified by the characteristic nuclear changes described and illustrated in Chapters 10 and 19. Kobayashi et al (1998) observed the pathognomonic cell changes in smears of only 4 of 11 patients in whom the diagnosis could be confirmed by culture and, in some cases, by in situ hybridization.
Moniliasis (Thrush)
Clinically, moniliasis forms a characteristic white coating of the oral cavity. This organism may be identified with ease by finding the characteristic fungal spores and pseudohyphae (see Chap. 10). This harmless infection, previously occurring mainly in debilitated patients and diabetics, has been recognized as one of the first manifestations of the acquired immunodeficiency syndrome (AIDS).
Blastomycosis
Sivieri de Araujo et al (2001) described the application of oral smears for diagnosis of Paracoccidiomycosis (South American blastomycosis), a common and serious disorder in Latin American countries. The yeast form is described in Chapter 19.
CHANGES IN ORAL SQUAMOUS CELLS IN DEFICIENCY DISEASES
In diseases associated with deficiencies in vitamin B12 and in folic acid, such as pernicious anemia, the squamous cells of the oral mucosa may show significant enlargement of both the nucleus and the cytoplasm (Graham and Rheault, 1954; Massey and Rubin, 1954; Boen, 1957). Similar changes may be observed in the related disorder, megaloblastic anemia (Boddington and Spriggs, 1959), and in tropical sprue (Staats et al, 1965). The findings were documented by comparison with normal cell populations and are statistically impressive. Vitamin B12 and folic acid are essential for DNA synthesis. If there is an insufficient supply of either factor, the DNA synthesis becomes disturbed, with resulting cell enlargement (Beck, 1964). There is evidence that this change is not confined to the oral epithelium but may affect many tissues (Foroozan and Trier, 1967). In reference to the uterine cervix, the changes were discussed in Chapter 17. For changes in the gastrointestinal tract, see Chapter 24.
In my own experience, the oral smears from patients with a variety of disorders, probably having malnutrition as a common denominator, may occasionally have a population of large squamous cells with vesicular nuclei and numerous chromocenters. The finding should lead to a hematologic work-up of the patients. A marked enlargement of squamous cells may also be caused by radiotherapy (see below).
Nieburgs described nuclear enlargement and “discontinuous nuclear membrane” in buccal squamous cells in 72% of patients with cancer. He considered this malignancy associated change (MAC) as reflecting “an altered mitotic function of cells.” Some of Nieburgs’ material was air-dried and the observations may be an artifact. The specific association of the buccal cell changes with cancer remains unproven. For further comments on MAC, see Chapter 7.
OTHER BENIGN DISORDERS
Benign Leukoplakia
Heavy keratin formation on the surface of oral epithelium is a common phenomenon occurring at the line of teeth occlusion, the palate, parts of gingiva, and occasionally elsewhere. The milky white appearance of such areas is
best classified clinically as leukoplakia and appears histologically as a benign squamous epithelium, topped with layers of keratin. This benign disorder must be differentiated from precancerous leukoplakia, which may have a similar clinical appearance. The differences are based on cytologic and histologic features, discussed in detail below.
best classified clinically as leukoplakia and appears histologically as a benign squamous epithelium, topped with layers of keratin. This benign disorder must be differentiated from precancerous leukoplakia, which may have a similar clinical appearance. The differences are based on cytologic and histologic features, discussed in detail below.
Cytology of benign leukoplakia is very simple, with fully keratinized, yellow or yellow-orange stained cells without nuclei (anucleated squames)




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree