Chapter 7 Occupational, environmental and iatrogenic lung disease
This chapter deals with the pneumoconioses, occupational asthma, occupational fevers and the effects o£ toxic fume and gases. Occupational diseases of the lung considered elsewhere include the effects of atmospheric pressure changes (see p. 368), extrinsic allergic alveolitis (see p. 279), carcinoma of the lung (see p. 534) and asbestos-induced pleural disease (see pp. 714–729). Dusty occupations in general are also associated with an increased risk of chronic obstructive pulmonary disease.1,2
Pneumoconiosis: general features
Definition of pneumoconiosis
The term ‘pneumoconiosis’ is an abbreviation (and etymological corruption) of Zenker’s pneumonokoniosis,3 which derives from pneumon (lung) and konis (dust) and therefore translates as dusty lung; in practice the term is confined to the effects of mineral dust on the lungs. Diseases caused by organic dusts are not included among the pneumoconioses and, in medicolegal practice at least, the presence of dust alone is insufficient to indicate pneumoconiosis: for compensation to be considered, the mineral dust must alter the structure of the lung and cause disability. The British Industrial Injuries Advisory Council defined pneumoconiosis as ‘permanent alteration of lung structure due to the inhalation of mineral dust and the tissue reactions of the lung to its presence, excluding bronchitis and emphysema’.4 Parkes recommends that cancer and asthma caused by mineral dust should also be excluded from the definition, an opinion with which we concur.5
Dust deposition in the lung
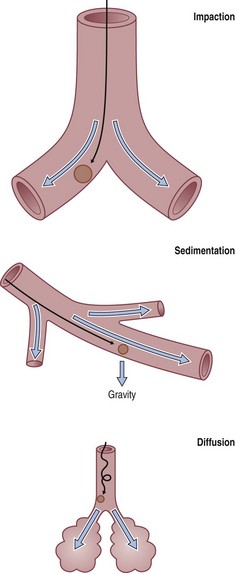
To reach the lung, dust particles have to be very small. Particle density and shape also affect the aerodynamic properties of dust. Host factors such as airflow characteristics, airway branching patterns and airway disease also affect dust deposition. Three deposition mechanisms are recognised (Fig. 7.1.1):
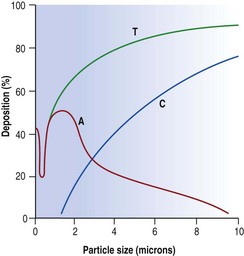
Inhaled dust particles are liable to sediment out in the alveoli if they have a diameter in the range of 1–5 µm, are roughly spherical in shape, and in density approximate to that of water. Larger or denser particles impact or precipitate on the walls of the conductive airways and are rapidly removed by ciliary action. Smaller particles may reach the alveoli but do not sediment so readily and many are therefore exhaled. Very small particles are deposited on the walls of alveoli by diffusion but because they are so small the total amount of dust deposited in this way is insignificant compared with that deposited by sedimentation (Fig. 7.1.2). Direct measurement shows that most lung dust (96%) has a particle diameter less than 2.5 µm.6
Fibrous dust particles behave differently. Fibres over 100 µm in length may reach the alveoli if they are very thin and remain aligned with the air stream. Fibre penetration is inversely related to path length and the number of bifurcations.7 Tall people have longer conductive airways and experience more deposition in these sites than short people who have greater alveolar deposition for the same level of exposure.8
Slightly more dust is deposited in the right lung than the left, probably because the right main bronchus is more in line with the trachea, and is broader and shorter than the left, and carries 55% of the inhaled air.9,10
Dust clearance from the lung
Inhaled dust that settles in the conductive airways is removed within a day or two by ciliary action. Only dust that reaches the alveoli is liable to cause pneumoconiosis and much of this is also removed, but the clearance rate here is much slower: many coalminers continue to expectorate mine dust years after retirement. Alveolar clearance is largely effected by macrophages, principally via the airways to the pharynx but also via lymphatics to the regional lymph nodes. The airway and interstitial routes interconnect at the bronchiolar level11 where some dust-laden macrophages leave the interstitium for the air space.12 This interconnection is probably the route utilised by circulating macrophages clearing other parts of the body of endogenous or exogenous particulate matter via the lung.13 Long asbestos fibres present a particular problem to macrophage clearance. Some minerals, notably chrysotile asbestos, undergo slow physicochemical dissolution in the lungs.
Only a small fraction of the inhaled dust gains access to the interstitium, a necessary step if it is to cause pneumoconiosis. Some free dust enters through the bronchus-associated lymphoid tissue11,12 and some is taken up by, or pierces, the alveolar epithelium (Fig. 1.34, p. 21).14–16 Some of this is transported within hours to the hilar lymph nodes.17 So rapid is this translocation that it is thought not to involve phagocytes, although interstitial macrophages are undoubtedly important in continuing the transportation of dust to the nodes. Ultrafine dust particles are particularly liable to be transported across the alveolar epithelium.11 The integrity of the alveolar epithelium is very important to dust translocation from the air spaces to the interstitium. Much more dust reaches the interstitium if the epithelium is damaged.18,19
It is widely thought that macrophages that have left the interstitium for the alveolar space never return,17,20 but this is probably untrue.21 Heavily laden macrophages accumulate in alveoli bordering the terminal and respiratory bronchioles, eventually filling them completely. Erosion of the alveolar epithelium permits re-entry of these macrophages into the interstitium,22 very close to foci of bronchial mucosa-associated lymphoid tissue (MALT), which are found near the terminal bronchioles.23 These aggregates guard the mouths of lymphatics, which commence at this point; alveoli are devoid of lymphatics. Dust-laden interstitial macrophages accumulate in and around the bronchial MALT, which Macklin therefore referred to as dust sumps.24 Most pneumoconiotic lesions are found in the region of the dust sumps and are therefore focal. Asbestosis is diffuse rather than focal because the long asbestos fibres are not readily mobilised and cannot be concentrated in the centriacinar dust sumps. This is also seen on occasion with platy non-fibrous dusts such as talc, mica, kaolinite and feldspar.25–33 Within the dust sumps the dust particles are not static. They are constantly being freed and reingested by interstitial macrophages and, because these cells are mobile, successively inhaled dusts soon become intimately mixed.34 Macrophages play an important role in pneumoconiosis and if the dust is fibrogenic the repeated phagocytosis of indestructible mineral particles results in constant fibroblast stimulation.
The zonal distribution of pneumoconiosis
Pneumoconiosis affects both lungs but seldom evenly and some pneumoconioses show characteristic patterns of lung involvement. In most, the lesions are more numerous and better developed in the upper lobes than the bases but the reverse is true of asbestosis. The reasons for this are complex but undoubtedly involve the dust deposition:clearance ratio for the effect of the dust will depend upon both its amount and the duration of its stay in the lungs. There are well-recognised regional differences in the distribution and clearance of inhaled material, which in turn are dependent upon man’s upright posture, the consequent gravitational forces being maximal at the apices.35 When standing at rest, the apices of the lungs are hardly perfused, so that lymph formation and clearance are much better at the bases.36–38 Similarly, the apices are relatively less well aerated; alveoli in the lower lobes receive more air than those in the upper lobes.37,39 The greater respiratory excursions at the bases are thought to promote macrophage mobility there. It is to be expected therefore that the bases would both receive and clear more dust than the apices, rendering it difficult to predict on theoretical grounds which parts of the lungs carry the heaviest dust burden. In fact, more dust of all types is found in the upper lobes, the part most severely affected by every type of pneumoconiosis except asbestosis.40,41 The predilection of asbestos to affect the periphery of the lower lobes is attributed to the dangerous long asbestos fibres preponderating there.41,42
Pulmonary reactions to mineral dust
The main tissue reaction to mineral dust is fibrosis. Silica is highly fibrogenic and is therefore very likely to cause pneumoconiosis. Carbon is non-fibrogenic and therefore, unless there are complications, coal pneumoconiosis causes little disability. Tin too is harmless, and stannosis therefore unimportant, although the chest radiograph is highly abnormal because tin is very radiopaque. Stannosis is one of several terms that specify pneumoconiosis due to a particular mineral, the best known being silicosis, asbestosis and anthracosis. Table 7.1.1 summarises the various pulmonary reactions to mineral dust.
Table 7.1.1 Pulmonary reactions to mineral dust
| Pulmonary reaction | Examples |
|---|---|
| Macrophage accumulation with a little reticulin deposition | |
| Nodular or massive fibrosis | |
| Diffuse fibrosis | |
| Epithelioid and giant cell granulomas | Chronic berylliosis |
| Alveolar lipoproteinosis | ‘Acute’ silicosis, but also seen with heavy exposure to other dusts (see p. 317) |
| Small-airway disease | Various dusts (see p. 123) |
Identification of the dust
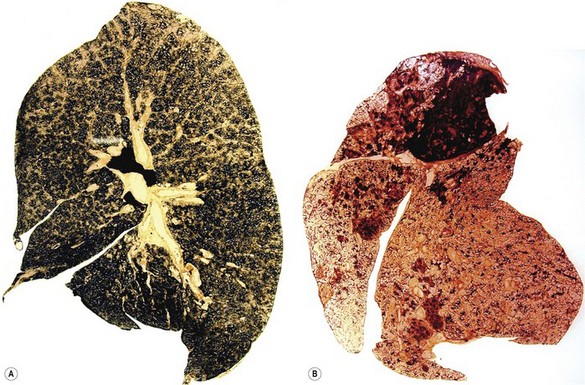
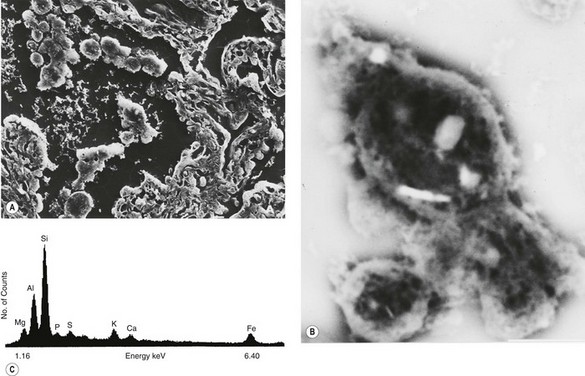
The blackness of carbon and red-brown colour of iron give ample evidence, both naked-eye and microscopically, of the type and amount of these dusts when they are present in the lung (Fig. 7.1.3), but other inorganic dusts may be more difficult to identify. However, a flick-out substage condenser and Polaroid filters to test for refractility and birefringence respectively are useful adjuncts that are too often neglected by the histopathologist. Crystalline silica is traditionally regarded as being only weakly birefringent, in contrast to silicates which generally show up brightly with simple crossed Polaroid filters.43 However, with modern microscope lamps, if the light source is set at high intensity when using Polaroid filters, both silica and silicates are birefringent.44 Mineralogists use polarising microscopy for analysis, but only by studying large polished crystals with controlled orientation of the light. The small dust particles found in tissue sections are too small to permit analysis by this technique but it is nevertheless very useful for detecting their presence (Fig. 7.1.4).
Microincineration combined with dark-field microscopy can also be used to demonstrate small particles. Incombustible mineral particles that cannot be seen with bright-field or polarising microscopy are rendered visible by this technique and their position on the slide can be compared with tissue reactions evident in a serial section that has not been incinerated. Microincineration has, however, also been largely replaced by modern analytical techniques that will now be considered.
Analytical electron microscopy is very helpful in identifying minerals, whether applied to lung digests or tissue sections.45–48 Scanning electron microscopy permits the examination of thicker sections than transmission electron microscopy but does not detect very small particles. However, scanning electron microscopy allows more tissue to be examined and avoids the difficulty of cutting mineral particles with an ultramicrotome.
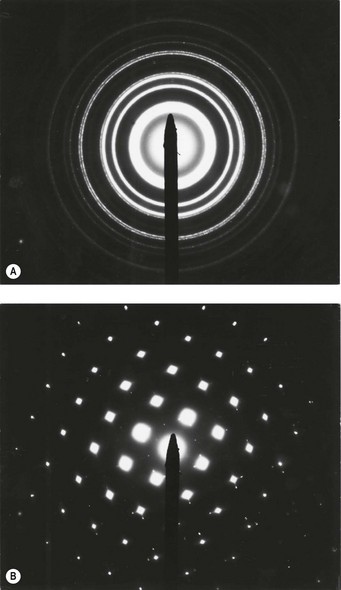
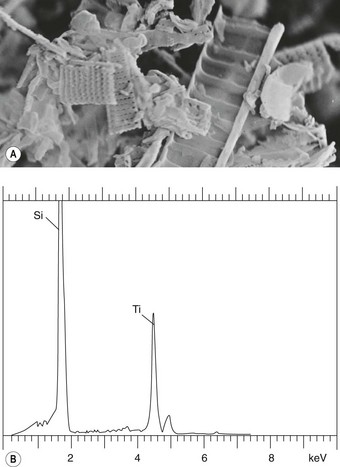
Mineral particles in a 5-µm thick deparaffinised section can be recognised in a scanning electron microscope set to collect the back-scattered electrons.46 The instrument can then be focused on points of potential interest and switched to X-ray diffraction, which provides information on crystal structure (Fig. 7.1.5). Alternatively, elemental analysis may be undertaken with either energy-dispersive or wavelength-dispersive X-ray spectroscopy. With energy-dispersive X-ray spectroscopy, all elements of atomic number above 11 are identified, whilst with wavelength-dispersive X-ray spectroscopy the section can be scanned for one particular element. With the former technique different elements are shown graphically as individual peaks, the heights of which are proportional to the amounts of the different elements within the particle studied, thereby giving information on probable molecular formula (Fig. 7.1.6). Thus, different silicates can be distinguished from each other and also from silica, which registers as pure silicon, oxygen (atomic number 8) not being detected. The fact that the elements of low atomic number that constitute organic chemicals are not detected means that any minerals present (except beryllium, atomic number 4) can be recognised easily in tissue sections. Only particles can be analysed however: elements present in only molecular amounts cannot be detected by X-ray analysis.
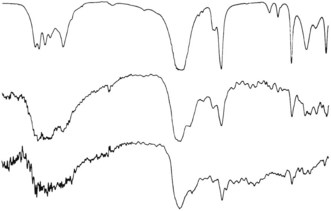
The detection of trace amounts of substances such as beryllium requires bulk chemical analysis or techniques that are not widely available such as atomic absorption spectrometry, neutron activation analysis and microprobe mass spectrometry.49,50 The last of these techniques can also provide molecular (as opposed to elemental) analysis of organic as well as inorganic particles.51 Another analytical technique of interest is microscopic infrared spectroscopy which provides data on the compound nature of microscopic particles in tissue sections (Fig. 7.1.7). Micro-Raman spectroscopy is also useful in this respect. Some metals cause hypersensitivity, which can be identified by exposing the patient’s lymphocytes to metals and measuring their reaction in vitro.52
Radiological grading of pneumoconiosis
A scheme for grading pneumoconiosis radiologically by comparison with standard radiographs has been adopted by the International Labour Organisation (ILO) and is widely used.53 Small opacities (up to 1 cm diameter) are graded by their profusion, 1, 2 and 3 indicating increasing numbers, and by their size, increasing through p, q and r if rounded and s, t and u if irregular. Type p opacities are described as punctiform and measure up to 1.5 mm in diameter; larger lesions up to 3 mm in diameter (type q) are described as micronodular or miliary; and those over 3 mm and up to 1 cm in diameter (type r) are described as nodular. Irregular opacities cannot be sized so accurately, s, t and u indicating fine, medium and coarse respectively. Large opacities (over 1 cm diameter) are graded by their combined size, increasing through A, an opacity measuring between 1 and 5 cm in diameter; B, one or more opacities whose combined area does not exceed the equivalent of one-third of the area of the right lung field (when they are regrouped in the mind’s eye or measured with a transparent ruler); and C, one or more opacities whose combined area exceeds one-third of the area of the right lung field (when similarly regrouped). In coalworkers, small opacities (up to 1 cm diameter) correspond to simple coalworker’s pneumoconiosis and large opacities (over 1 cm diameter) to complicated coalworker’s pneumoconiosis, which is also known as progressive massive fibrosis.
Silicosis43
Mineralogy
Crystalline silica is highly fibrogenic whereas amorphous silica and silicates other than asbestos are relatively inert. Silica exists in several crystalline forms, of which quartz, cristobalite and tridymite are the most important: tridymite is the most fibrogenic and cristobalite more so than quartz.43,54
Occupations at risk
Silica in one form or another is used in many trades – in the manufacture of glass and pottery, in the moulds used in iron foundries, as an abrasive in grinding and sandblasting, and as a furnace lining that is refractory to high temperatures. Rocks such as granite and sandstone are siliceous and their dusts are encountered in many mining and quarrying operations. In coal mining in the UK the highest incidence of the disease was in pits where the thinness of the coal seams required the removal of a large amount of siliceous rock, a process known as ‘hard heading’. In South Africa, silicosis causes a high mortality among the gold miners on the Witwatersrand, where the metallic ore is embedded in quartz. Slate is a metamorphic rock that contains both silica and silicates, and slateworkers develop both silicosis and mixed-dust pneumoconiosis.55,56 Nor are rural industries immune from the disease, particularly if ventilation is inadequate, as it is in certain African huts where stone implements are used to pound meal and the occupants develop mixed-dust pneumoconiosis.57 Silicosis and mixed-dust pneumoconiosis have also been reported in dental technicians.58
Desert sand is practically pure silica but the particles are generally too large to reach the lungs. However, silicosis has been reported in inhabitants of the Sahara, Libyan and Negev deserts and those living in windy valleys high in the Himalayan mountains,59–65 whilst in California the inhalation of dust raised from earth has led to silicate pneumoconiosis in farm workers,66 horses67 and a variety of zoo animals.68
Clinical features
Silicosis sometimes develops more rapidly, perhaps within a year or so of first exposure. Such ‘acute silicosis’ was observed in the scouring powder industry in the 1930s when these cleansing agents consisted of ground sandstone mixed with a little soap and washing soda.69,70 The additives were considered to have rendered the silica in the sandstone more dangerous but it is possible that the rapidity of onset of the disease merely reflected the intensity of the dust cloud to which the packers were exposed. Confusingly, the term ‘acute silicosis’ has since been applied to a further effect of heavy dust exposure in tunnellers, sand blasters and silica flour workers, namely pulmonary alveolar lipoproteinosis (see below),71,72 whilst the terms ‘accelerated silicosis’ or ‘cellular phase silicosis’ have been substituted for ‘acute silicosis’ in referring to the rapid development of early cellular lesions.43,73
Pathological findings
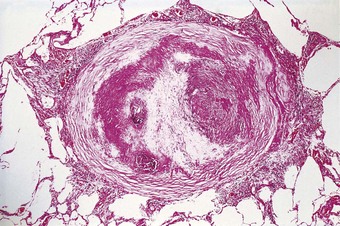
Silica particles that are roughly spherical in shape and of a diameter in the range of 1–5 µm sediment out in the alveoli and are concentrated within macrophages at Macklin’s dust sumps, as explained previously (see p. 27). Early lesions, as seen in so-called accelerated or cellular phase silicosis, consist of collections of macrophages separated by only an occasional wisp of collagen. The early lesions have been likened to granulomas and on occasion have been mistaken for Langerhans cell histiocytosis or a storage disorder, but Langerhans cells are scanty and the histiocytes contain dust particles rather than accumulated lipid or polysaccharide. The macrophages of the early lesion are gradually replaced by fibroblasts and collagen is laid down in a characteristic pattern. The mature silicotic nodule is largely acellular and consists of hyaline collagen arranged in a whorled pattern, the whole lesion being well demarcated (Fig. 7.1.8) and sometimes calcified. Small numbers of birefringent crystals are generally evident within the nodules when polarising filters are used, but these mainly represent silicates such as mica and talc, inhaled with the silica. Silica particles are generally considered to be only weakly birefringent,43 but fairly strong birefringence is evident in strong light (see above).44
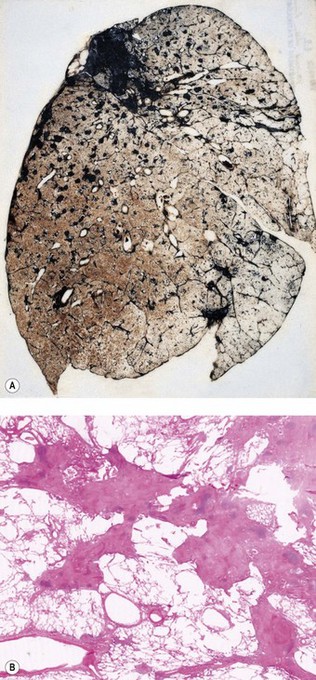
The silicotic nodules are situated in the centres of the pulmonary acini and are more numerous in the upper zones than the bases (Fig. 7.1.9A). They measure up to 5 mm across and are hard and easily palpable. They are grey if caused by relatively pure silica but black in coalminers and red in haematite miners.
Silicotic nodules develop first in the hilar lymph nodes and are generally better developed there than in the lungs.74–76 Indeed, silicotic nodules are occasionally found in the hilar lymph nodes of persons who have no occupational history of exposure to silica and whose lungs are free of such lesions, the silica in the nodes being presumed to represent inhaled particles derived from quartz-rich soil.77 Severely affected lymph nodes often calcify peripherally, giving a characteristic eggshell-like radiographic pattern. This is sometimes the only radiological abnormality.76 Such enlarged lymph nodes may occasionally press upon and obstruct adjacent large bronchi78 or result in a left recurrent laryngeal nerve palsy,79 so simulating malignancy.80 Sometimes the nodules develop within the walls of major bronchi, occasionally causing a middle-lobe syndrome (see p. 92).81 Silicotic nodules are also found along the lines of the pleural lymphatics75,82 where they have been likened to drops of candle wax on the visceral pleura. Very rarely, silica-induced fibrosis is more pronounced in the pleura than in the lungs.83
Lung tissue between the nodules is often quite normal and not until the process is very advanced is there any disability (Fig. 7.1.9B). In severe cases large masses of fibrous tissue are formed, which may undergo central necrosis and cavitation (Fig. 7.1.10).84 On close inspection it is evident that these consist of conglomerations of many silicotic nodules closely packed together. In such severe cases cor pulmonale develops. Occasionally, silicotic nodules develop in the abdominal as well as the thoracic lymph nodes, and in the liver, spleen, peritoneum and bone marrow.85–89
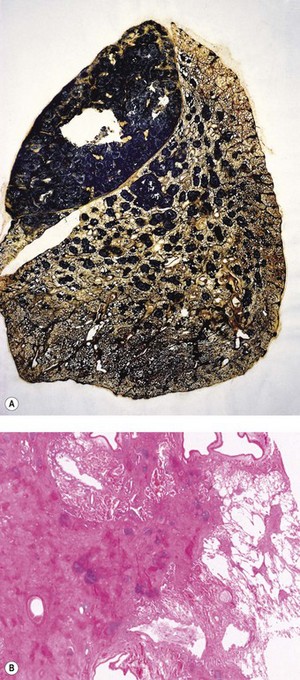
Figure 7.1.10 Silicosis. (A) The nodules are larger than in Figure 7.1.9a and have fused together. Cavitating conglomerate silicosis destroys most of the upper lobe. Paper-mounted whole-lung section. (B) Microscopy of conglomerate silicosis.
In about 10% of cases, the typical pulmonary nodules that predominantly affect the upper lobes are accompanied by diffuse fibrosis that is maximal in the lower lobes.27,33,90–92 The latter may show ‘honeycombing’ and closely resemble idiopathic pulmonary fibrosis. The association is too common to be explained by chance and the diffuse fibrosis is therefore regarded as a further manifestation of the pneumoconiosis, possibly due to an interaction between the dust and the immunological factors discussed below.
Pathogenesis
The pathogenesis of silicosis has excited much interest and many different theories have been advanced over the years. An early theory held that the hardness of the silica was responsible, but this was discounted by the observation that silicon carbide (carborundum) is harder than silica but is non-fibrogenic. Theories based on the piezoelectric property and on the solubility of silica were successively abandoned although the latter had a long period of popularity. It gained support from Kettle’s experiments which showed that fibrosis developed about chambers placed in an animal’s peritoneal cavity if the chambers contained silica powder sealed in by a collodion membrane through which solutes such as silicic acid could pass. However, it was later shown that the pores in a collodion membrane are quite irregular in size and when the experiments were repeated using chambers guarded by millipore membranes, no fibrosis developed, despite solutes being able to diffuse out.93 The solubility theory also fails to take account of the differing fibrogenicity of the various forms of silica despite them being of similar solubility.54 Furthermore, if the outer, more soluble layer of the particles is removed by etching, fibrogenicity is increased although solubility is decreased. In line with this, freshly fractured crystalline silica is more pathogenic in every respect than its aged equivalent,94 which may partly explain the severity of silicosis in trades such as sandblasting. These observations suggest that the fibrogenicity of silica is connected with its surface configuration.
It is now known that uptake of the silica by macrophages is necessary for silicosis to develop. If silica and macrophages are enclosed together in peritoneal millipore chambers, a soluble product of the macrophages diffuses out and causes fibrosis. This observation led to the realisation that the fibrogenicity of the various crystalline forms of silica correlated well with their toxicity to macrophages and for a time macrophage death was thought to be necessary.95 It is now considered that before the macrophages are killed by the ingested silica, they are stimulated to secrete factors that both damage other constituents of the lung and promote fibrosis.96–102 Transforming growth factor-β is one fibrogenic factor that has been implicated in the pathogenesis of silicosis.103–105
Toxic damage to macrophages is due to silica particles injuring the phagolysosomal membranes, so releasing acid hydrolases into the cytoplasm.95 It is important in the pathogenesis of the disease indirectly because when the macrophage crumbles, the silica particles are taken up by fresh macrophages and the fibrogenic process continues. It has been suggested that early involvement of the hilar lymph nodes in the fibrogenic process promotes the development of the disease in the lung by delaying dust clearance.74
Immunological aspects of silicosis
Immunological factors have been implicated in the pathogenesis of silicosis because many patients with silicosis have polyclonal hypergammaglobulinaemia, rheumatoid factor or antinuclear antibodies, and because there is a well-recognised association between autoimmune diseases such as systemic sclerosis and rheumatoid disease and exposure to silica.43,106–109 The relation of immunity to dust exposure appears to be a reciprocal one: on the one hand, the presence of dust results in rheumatoid lesions in the lungs being more florid (see Caplan’s syndrome, p. 341), whilst on the other, non-specific immunisation of rabbits with horse serum results in experimental silicotic lesions being larger and more collagenous.110 It is doubtful whether pneumoconiosis and autoimmune disease play a causative role in each other but one seems to aggravate the other and may lead to its earlier development.
Tuberculosis complicating silicosis (silicotuberculosis)
One of the commonest and most feared complications of silicosis is chronic respiratory tuberculosis.109 Once this infection has been added to the silicosis, the prognosis rapidly worsens. It is thought that in the presence of silica, the tubercle bacilli proliferate more rapidly because the ingested silica particles damage phagolysosomal membranes and thereby interfere with the defensive activity of the macrophages. The synergistic action of silica dust has long been held responsible for the inordinately high incidence of respiratory tuberculosis in mining communities. Many former South African gold miners now have acquired immunodeficiency syndrome (AIDS) as well as silicosis and tuberculosis has consequently reached almost epidemic proportions amongst these men. Phagocyte damage by ingested dust particles may also cause some cases of chronic necrotising aspergillosis complicating pneumoconiosis.111
Silica-induced lung cancer
A series of studies suggesting that there might be a link between silica inhalation and lung cancer was reviewed by the International Agency for Research on Cancer in 1987, leading to the conclusion that the evidence for carcinogenicity of crystalline silica in experimental animals was sufficient, while in humans it was limited.112 Subsequent epidemiological publications were reviewed in 1996, when it was concluded that the epidemiological evidence linking exposure to silica to the risk of lung cancer had become somewhat stronger113 but that in the absence of lung fibrosis remained scanty.113 The pathological evidence in humans is also weak in that premalignant changes around silicotic nodules are seldom evident.114 Nevertheless, on this rather insubstantial evidence, lung cancer in the presence of silicosis (but not coal or mixed-dust pneumoconiosis) has been accepted as a prescribed industrial disease in the UK since 1992.115 Some subsequent studies have provided support for this decision.116 In contrast to the sparse data on classic silicosis, the evidence linking carcinoma of the lung to the rare diffuse pattern of fibrosis attributed to silica and mixed dusts is much stronger and appears incontrovertible.33,92
Alveolar lipoproteinosis in response to heavy dust exposure
A further complication of exposure to silica is the development of alveolar lipoproteinosis (see p. 317).71,72,117,118 Very heavy experimental exposure to silica, and indeed other dusts, stimulates hypersecretion of alveolar surfactant to such an extent that the normal clearance mechanism is overwhelmed.119–125 Alveolar macrophages are enlarged by numerous phagolysosomes distended by lamellar bodies that represent ingested surfactant. The alveoli are filled by such cells and, having a foamy cytoplasm, they produce the appearances of endogenous lipid pneumonia, similar to that more usually encountered as part of an obstructive pneumonitis distal to a bronchial tumour. The macrophages gradually disintegrate and the free denatured surfactant slowly becomes compacted, during which time its staining with both eosin and the periodic acid–Schiff reagents intensifies until the appearances are finally those of alveolar lipoproteinosis. This process prevents the aggregation and concentration of the dust in the usual foci and thereby hinders the development of silicosis. Lipoproteinosis and silicosis may be seen in conjunction but, more often, different areas of the lung show one or the other. The lipoproteinosis has its own severe impact on lung function, but, unlike silicosis, is potentially reversible (by massive alveolar lavage).
Silica-induced renal disease
Occasional patients exposed to silica develop renal disease.126–129 Two mechanisms appear to operate. First, translocation of silica particles from the lungs leads to their deposition in the renal interstitium with resultant nephrotoxity. Second, silica stimulates an autoimmune response characterised by the formation of various antibodies, notably rheumatoid factor and antinuclear antibodies, which leads to the development of immune complex-mediated glomerulonephritis.128,129
Amorphous silica
Manmade submicron forms of silica, variously known as amorphous, vitreous, colloidal, synthetic or precipitated silica, are widely used in industry. They consist of pure non-crystalline silicon dioxide. Particle size ranges from 5 to 200 nm but aggregates of the particles measure from 1 to 10 µm. Industrial surveys suggest that inhalation of such dust is harmless, observations that are in accord with the results of animal experiments.54
Diatomaceous earth (keiselguhr)
An amorphous silica is the principal component of the fossilised remains of diatoms that constitute the sedimentary rock, diatomite (Fig. 7.1.11). This is generally obtained by open-cast mining, following which the rock is crushed and calcined. The calcined product is used in filters, insulation material and as a filler. Being amorphous, the silica in diatomite is harmless, but calcining (>1000°C) results in its conversion to crystalline forms of silica. Diatomaceous earth pneumoconiosis is unusual and its risk appears to be related to the amount of cristobalite and tridymite (two forms of crystalline silica) produced in the calcining process.130
Silicates43
The silicates are complex compounds in which silicon and oxygen form an anion combined with cations such as aluminium and magnesium: talc, for example, is a hydrated magnesium silicate with the formula Mg3Si4O10(OH)2. Silicates include fibrous forms (asbestos and the zeolites), plate-like forms (talc and mica) and clays (kaolinite and fuller’s earth). In histological sections, the platy talc and mica particles are generally cut tangentially and therefore appear needle-shaped (see Fig. 7.1.4). They are strongly birefringent whereas the clays are only weakly so. Talc particles in the lung exceeding 5 µm in length should arouse suspicion of intravenous drug abuse.131
Pneumoconiosis has been described with various non-fibrous silicates, notably in the rubber industry, which uses talc and, less commonly, mica as lubricants. Other occupations posing a risk include the extraction of kaolinite from china clay (kaolin),27,132,133 and in the open-cast and underground mining of fuller’s earth (montmorillonite, bentonite and attapulgite clays, which were originally used in ‘fulling’ (degreasing) wool).134,135 However, all these substances are commonly contaminated with silica, asbestos or both, and it has been questioned whether in pure state they are at all fibrogenic. The modifying effect of inert substances such as iron on that of silica is well known (see mixed-dust pneumoconiosis, below) and it has been suggested that talc, mica and fuller’s earth act in a similar way in regard to their more fibrogenic contaminants, the pneumoconioses attributed to them in reality representing mixed-dust pneumoconiosis or asbestosis. Contrary evidence comes from reports of pulmonary fibrosis in persons heavily exposed to pure talc, mica or kaolin.32
All these silicates are evident in the tissues as plate-like birefringent crystals which often provoke a foreign-body giant cell reaction (see Fig. 7.1.3) and may result in fibrotic nodules. Large focal lesions resembling the progressive massive fibrosis of coalworkers may be produced, and also a diffuse ‘asbestosis-like’ form of pneumoconiosis, the latter attributed to poor macrophage mobilisation of the plate-like particles.27–32,132–138 It would appear therefore that silicates are indeed fibrogenic if enough is inhaled; they appear to vary in fibrogenicity but in all cases they are less fibrogenic than silica.
Inert dusts
Inert dusts are non-fibrogenic and therefore of little clinical consequence, although elements of high atomic number can give rise to a striking chest radiograph.139 It should be noted however that inert or lowly fibrogenic materials may be associated with substances of medical importance, for example, kaolin, bentonite and barytes (barite) may all be contaminated with silica27,134,140 and talc may be contaminated with asbestos.
Carbon deposition is commonly found in the lungs, particularly those of city dwellers and tobacco smokers. It is also the principal constituent of coal, which is dealt with separately below, and large amounts of pure carbon may be inhaled by workers involved in the manufacture of carbon black, carbon electrodes and charcoal.141–144 Although carbon is regarded as being non-fibrogenic, the very heavy lung burdens encountered in industries such as these may lead to the complicated form of pneumonconiosis known as progressive massive fibrosis that is more commonly encountered in coal workers (see p. 340). Heavy pure carbon deposition may also be acquired domestically when wood is burnt in buildings devoid of a chimney, so-called ‘hut lung’,145 a term that is also applied to the domestic acquisition of carbon mixed with silica or silicates, resulting in forms of mixed-dust pneumoconiosis.57,64,65
Anthracofibrosis is a term introduced by Chinese bronchoscopists for bronchial stenosis or obliteration associated with carbon pigmentation of the mucosa.146 Although the original description incriminated tuberculosis, mixtures of various mineral dusts acquired at work or domestically are a more likely cause.147–150
Iron dust in the lungs was first described by Zenker in 1867, when he also introduced the terms siderosis and pneumonokoniosis.3 Zenker was describing a woman who coloured paper with iron oxide powder (‘rouge’), a substance which is still encountered by some workers engaged in polishing silver, glass, stone and cutlery. Siderosis is also found in welders, iron foundry fettlers, steel workers, boiler scalers and haematite miners and crushers. Iron dust particles are reddish-brown but in the lung may be masked by carbon151: when evident, or revealed by microincineration, they resemble haemosiderin and generally give a positive Perls’ reaction, but particularly with haematite, heat (60–80oC) and concentrated (12N) hydrochloric acid may be necessary.152
Other inert dusts include barium, which also has a high atomic number and is therefore radiopaque,139 and minerals of low radiodensity such as limestone, marble and cement (all chiefly composed of calcium carbonate) and gypsum (hydrated calcium sulphate). However, the extraction of barium ore (almost entirely in the form of barium sulphate, which is known as barytes in Europe and barite in the USA) may entail exposure to silica and silicates. Pure baritosis resembles stannosis and siderosis.
Mixed-dust pneumoconiosis
The term ‘mixed-dust pneumoconiosis’ refers to the changes brought about by inhaling a mixture of silica and some other less fibrogenic substance such as iron, carbon, kaolin or mica.33,151,153–155 The proportion of silica is usually less than 10%. Typical occupations include foundry work and welding and the mining of coal, haematite, slate, shale and china clay.
Coal pneumoconiosis156
The term ‘anthracosis’ was initially applied to changes observed in a coalminer’s lung157 but is now often extended to include the common carbon pigmentation of city dwellers’ lungs, and the term ‘coal pneumoconiosis’ is more appropriate to a special form of pneumoconiosis to which coalworkers are subject, particularly those who work underground. The principal constituent of coal, carbon, is non-fibrogenic, so suspicion has naturally fallen on the ash content of mine dust, some of which derives from the coal, some from adjacent rock strata and some from stone dust laid in the roadways to minimise the risk of coal dust explosions. Coal itself appears to be the responsible agent because coal-trimmers, working in the docks and not exposed to rock dust, also develop the disease.158 Coalminers encountering siliceous rock are, of course, also liable to develop silicosis like other underground workers.
Mineralogy
In high-rank British collieries the development of coal pneumoconiosis appears to depend on the total mass of dust inhaled, whereas in low-rank British collieries the mineral content of the lung dust appears to be more important.159 This may explain apparently contrary data drawn from different coalfields – data based on coals of different composition that are not strictly comparable. Some workers have stressed the importance of silica in the dust whereas others, particularly in the high-rank coalfields of south Wales, have been unable to detect any association between silica and the level of pneumoconiosis. Both findings may be correct, but only for the particular group of miners examined in each case.160
Pathology
The lesions of coal pneumoconiosis are generally focal and fall into one or other of two major types, simple and complicated, depending upon whether the lesions measure up to or over 1 cm; simple corresponds to categories 1–3 of the ILO grading system (see p. 331) and complicated, which is also known as progressive massive fibrosis, to ILO categories A–C. More diffuse interstitial fibrosis has been reported in about 16% of Welsh and West Virginian coalminers, usually involving those carrying a particularly heavy dust burden; it runs a more benign course than non-occupational interstitial fibrosis (idiopathic pulmonary fibrosis).161 Similar findings have been reported from France.162
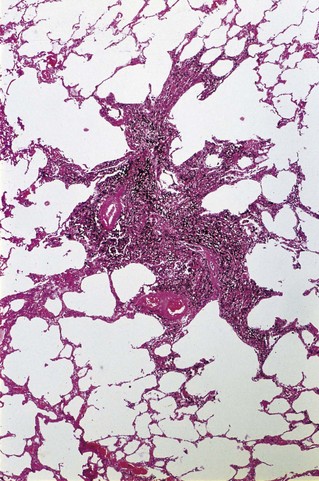
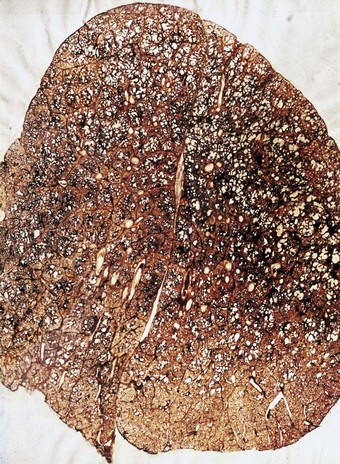
Simple coal pneumoconiosis consists of focal dust pigmentation of the lungs, which may be associated with a little fibrosis and varying degrees of emphysema. Its clinical effects are relatively minor. Some degree of black pigmentation (anthracosis) of the lungs is common in the general urban population, especially in industrial areas, but much denser pigmentation is seen in coalminers, whose lungs at necropsy are black or slate-grey. Black pigment is evident in the visceral pleura along the lines of the lymphatics and on the cut surface where it outlines the interlobular septa and is concentrated in Macklin’s centriacinar dust sumps (Fig. 7.1.12). The dust is generally more plentiful in the upper parts of the lungs and in the hilar lymph nodes, possibly due to poorer perfusion and consequently poorer lymphatic drainage there (see p. 21).162
Macules consist of closely packed dust particles, free or within heavily laden macrophages, so that the lesion appears black throughout (Fig. 7.1.13). Appropriate stains show that the dust-laden macrophages and free dust are lightly bound by reticulin. Very little collagen is evident. Although striking in their appearance, dust macules are thought to have little effect on lung function.
Nodules contain substantial amounts of collagen and are thought to have an adverse, but limited, effect on respiration. They vary from a heavily pigmented, stellate lesion, which apart from its collagen content resembles the dust macule (Fig. 7.1.14), to one that is less pigmented and more circumscribed. The stellate, heavily pigmented type of nodule is seen in lungs that have a relatively low ash content whilst the more rounded and less pigmented nodule is seen in lungs with relatively high ash loads.163
Radiologically (see p. 321), p-type opacities correspond to macules, q-type opacities to the stellate nodules that resemble those of mixed-dust pneumoconiosis and r-type opacities to the rounded nodules that resemble those of silicosis.53,164 Thus, the radiological changes of simple coalworker’s pneumoconiosis are due to the dust and the small amount of collagen present and do not reflect any emphysema that may also be present. However, pulmonary dust foci are often associated with emphysema (Fig. 7.1.15) and the severity of the emphysema appears to correlate with the dust load. The prevalence of chronic bronchitis and emphysema is high in the coal industry and it has long been debated whether occupation or cigarette smoking is the major factor contributing to emphysema in coalminers.165–168 As well as mineral dust, nitrous fumes from shot-firing form another occupational hazard of coal mining.
Heppleston made a special study of the emphysema found in coalminers, claiming that it differs from centriacinar emphysema, as seen in smokers in the general population, and attributing it to the dust.169 He introduced the term ‘focal emphysema of coalworkers’ to describe this special process. Others find it very difficult to identify any convincing difference between the emphysema of coalworkers and that encountered outside the industry but Heppleston based his claims on the study of serial sections. By this means he showed that, although both forms affect respiratory bronchioles, the focal emphysema of coalworkers affects more proximal orders of these airways and is not associated with the bronchiolitis seen with centriacinar emphysema. Furthermore, focal emphysema is a dilatation lesion whereas centriacinar emphysema involves destruction of adjacent alveolar walls. By definition, therefore, focal emphysema is not a true emphysema at all (see p. 102). However, it has been shown that mineral dusts cause elastin and collagen breakdown in the rat lung.170 Focal emphysema may progress to the destructive centriacinar form and this has strengthened claims that mine dust plays a causal role in centriacinar emphysema.1,171–176 In the UK, these claims have been accepted and chronic bronchitis and emphysema in coalminers and metal production workers have been accepted as prescribed industrial diseases since 1992.177 In Germany too, chronic obstructive pulmonary disease is now compensatable as an occupational disease. The conditions for compensation in the UK were initially:
However the last of these criteria has now been dropped. The inclusion of a time element and the omission of some estimate of dust load (such as radiological category) have been criticised, with some justification.178 As with lung cancer caused by chromates benefit is paid irrespective of smoking habits.
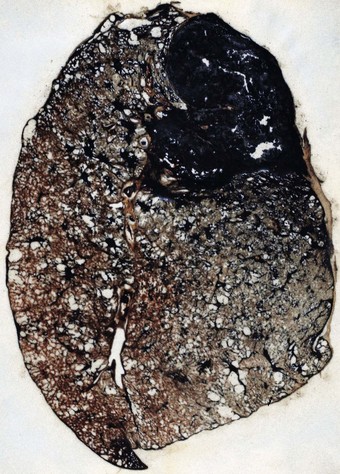
Whereas simple coal pneumoconiosis, particularly the macular variety, has little effect on lung function, complicated coal pneumoconiosis, also known as progressive massive fibrosis, can have very serious consequences. Particularly when the lesions are large, it is associated with productive cough, breathlessness, significant impairment of lung function and premature death. The major factor accounting for the development of progressive massive fibrosis appears to be the sheer bulk of coal dust in the lung, rather than coal rank or the silica content of the mine dust.179 Progressive massive fibrosis has occasionally been recorded in dockers loading silica-free coal into the holds of ships158 and in workers exposed to pure carbon in the manufacture of carbon black and carbon electrodes.141–143
Progressive massive fibrosis is characterised by large (over 1 cm) black masses, situated anywhere in the lungs but most common in the upper lobes. The lesions may be solitary or multiple and very large, occupying most of the lobe and even crossing an interlobar fissure to involve an adjacent lobe (Figs 7.1.3B, 7.1.16). They cut fairly easily, often with the release from a central cavity of black fluid flecked by cholesterol crystals. For many years it was believed that the condition was the result of synergism between mycobacterial infection and dust but the failure of the attack rate to decrease as tuberculosis declined negated this view.180 Today, more emphasis is placed on total dust load for the lesions tend to affect lungs that carry an unduly heavy dust burden. If the remainder of the lung shows little evidence of dust accumulation, the possibility of the masses representing Caplan-type lesions (see below) should be considered.
Microscopically, the lesions consist of dust and connective tissue intermixed in a random fashion. Central necrosis and cavitation commonly occur. The necrosis is thought to be ischaemic.181 It is amorphous or finely granular, and eosinophilic apart from abundant dust particles and cholesterol crystal clefts. The fibrotic component in a complicated pneumoconiotic lesion is rich in fibronectin, with collagen only more abundant at the periphery.182 Two types of progressive massive fibrosis are recognisable, corresponding to the two types of nodule described in simple coal pneumoconiosis.163 The first appears to have arisen by enlargement of a single nodule, whereas the second is a conglomeration of individual lesions, each of which corresponds to the more circumscribed type of nodule seen in simple coal pneumoconiosis. The ash content of the lungs bearing these two types of progressive massive fibrosis varies in the same way as with the two types of simple pneumoconiotic nodules, the enlarged single lesion being found in lungs with a relatively low ash content, and the conglomerate lesion in lungs with a relatively high ash content. The second type resembles the conglomerate nodules of large silicotic lesions but lacks the characteristic whorled pattern of the latter.
The diffuse interstitial fibrosis found in a minority of coalworkers is associated with heavy dust deposition. It may progress to honeycombing but, as with the focal forms and unlike idiopathic interstitial fibrosis, it is better developed in the upper zones, the reasons for which are discussed above (see the zonal distribution of pneumoconiosis, p. 329).
Pathogenesis
The pathogenesis of coal pneumoconiosis has much in common with that of silicosis, and indeed many other pneumoconioses. It involves the promotion of fibrogenic factor synthesis and release by cells phagocytosing the inhaled dust. Several such factors have now been identified, the degree of fibrosis produced varying with the amount of dust inhaled and the ability of its constituents to promote the production of the responsible cytokines. These include platelet-derived growth factor, insulin-like growth factors 1 and 6, transforming growth factor-β and tumour necrosis factor-α.101,183,184 As with other minerals, the indestructability of the dust perpetuates the process.
As in silicosis, immunological factors appear to be involved, for there is an increased prevalence of rheumatoid arthritis185 and of circulating autoantibodies186–188 in miners with coal pneumoconiosis. Rheumatoid factor has also been demonstrated within the lung lesions.189 These abnormalities are generally more pronounced in miners with complicated pneumoconiosis but are also found in those with the simple variety. It is also possibly pertinent to the immunological basis of coal pneumoconiosis that some of the pulmonary manifestations of rheumatoid disease are more pronounced in coalminers. This was first pointed out by Caplan and will be considered next.
Pneumoconiosis and rheumatoid disease (Caplan’s syndrome)
Caplan described distinctive radiographic opacities in the lungs of coalminers with rheumatoid disease,190 and it is now recognised that similar lesions may develop in rheumatoid patients exposed to siliceous dusts. The development of such rheumatoid pneumoconiosis does not correlate with the extrapulmonary or serological activity of the rheumatoid process. Nor is there a strong relation to dust burden: Caplan lesions are characteristically seen in chest radiographs that show little evidence of simple coal pneumoconiosis.
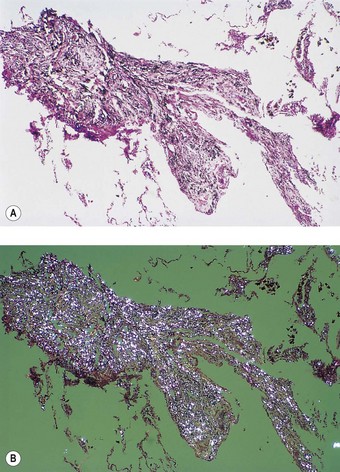
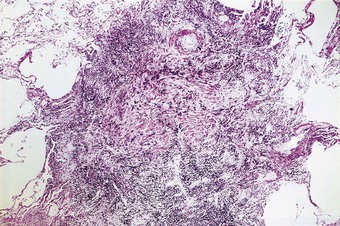
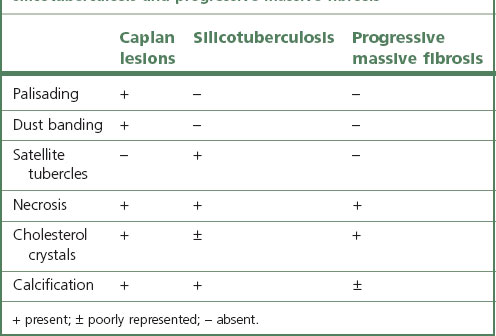
Pathologists recognise the lesions as particularly large necrobiotic nodules similar to those seen in rheumatoid patients who are not exposed to dust (Fig. 7.1.17). However, because of their large size (up to 5 cm diameter) they may be confused with progressive massive fibrosis undergoing central ischaemic necrosis (see above) or silicosis complicated by caseating tuberculosis. Such errors will be less likely if the radiological evolution of the lesions is considered for they tend to cavitate and undergo rapid remission, only to be succeeded by others. They are also well demarcated radiologically. Pathologically, they resemble rheumatoid nodules in showing peripheral palisading but differ in their large size and the presence of dust.191 The dust accumulates in circumferential bands or arcs within the necrotic centres of the lesion (Fig. 7.1.18), an arrangement that suggests periodic episodes of inflammatory activity. Caplan lesions differ from tuberculosis in lacking satellite lesions and tubercle bacilli, and from progressive massive fibrosis in showing characteristic bands of dust pigmentation (Table 7.1.2) and only light dust deposition in the surrounding lung.
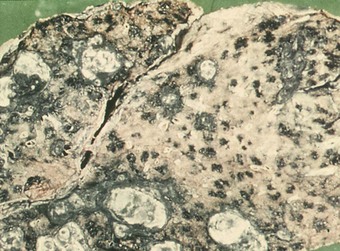
Figure 7.1.17 Caplan lesions in a coalminer’s lung, which characteristically shows only light dust deposition.
(Courtesy of Dr R Seal, formerly of Penarth, UK.)
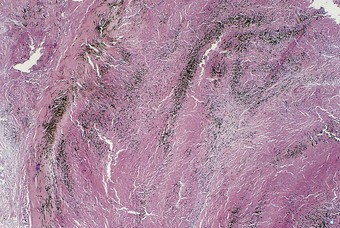
Figure 7.1.18 A Caplan lesion, characterised by successive bands of dust within the centre of a large necrobiotic nodule.
Asbestosis
Asbestosis is defined as diffuse interstitial fibrosis of the lung caused by exposure to asbestos dust.192,193 It does not cover asbestos-induced carcinoma of the lung or asbestos-induced pleural disease. The development of asbestosis depends on the presence of fairly large dust burdens: this is in contrast to mesothelioma and other forms of asbestos-induced pleural disease, which, although also dose-related, occur following the inhalation of far smaller amounts of asbestos dust.
Asbestos types and production
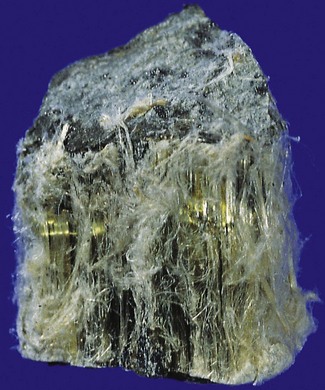
Chrysotile (white asbestos) is the only important serpentine form. It accounts for most of the world production of asbestos of all types (Table 7.1.3).194 Being a serpentine mineral, chrysotile consists of long, curly fibres that can be carded, spun and woven like cotton (Fig. 7.1.19). The curly chrysotile fibres are carried into the lungs less readily than the straight amphibole asbestos fibres, and once there undergo physicochemical dissolution and are cleared more readily. They readily fragment into short particles that are easily ingested by macrophages and in the acidic environment of the macrophage phagolysosome they are particularly unstable. The half-life of chrysotile in the lungs is estimated to be in the order of only a few months.195,196 Not surprisingly therefore chrysotile is the least harmful type of asbestos in respect of all forms of asbestos-induced pleuropulmonary disease.197–199 It may nevertheless cause pulmonary fibrosis if sufficient is inhaled.200,201
Table 7.1.3 Asbestos production by country, 2000194
| Country | Tons |
|---|---|
| Russia | 752000 |
| China | 350000 |
| Canada | 320000 |
| Brazil | 209000 |
| Kazakhstan | 179000 |
| Zimbabwe | 152000 |
| Others | 88000 |
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree