and Natalia Buza1
(1)
Department of Pathology, Yale University School of Medicine, New Haven, CT, USA
Keywords
Uterine epithelial neoplasmsTumorlike lesionsEndometrial hyperplasiaEndometrioid adenocarcinomaSerous carcinomaClear cell carcinomaUndifferentiated carcinomaNeuroendocrine carcinomaMetastatic carcinomaIntroduction
Among endometrial epithelial lesions, common nonneoplastic conditions (metaplasia, hormone-related changes, endometrial polyp, and gestational alterations) can mimic malignant or premalignant lesions of the endometrium. Endometrial carcinoma is the most common cancer of the female genital tract [1]. Long-term estrogen overload due to obesity, hormone replacement, oral contraceptives, and smoking are significantly associated with the most common subtype, endometrioid adenocarcinoma (type 1 endometrial cancer, 70–80 %) [2] and its precursor lesion—atypical endometrial hyperplasia. A smaller subset of endometrial cancers (10–15 %) is represented by serous and clear cell carcinomas (type 2 endometrial cancer) [3] that are high grade by definition and almost always occur in postmenopausal women. Mixed carcinoma is defined by the presence of two histological subtypes of epithelial malignancy, one of which is type 2 (serous or clear cell carcinoma) and the minor component constitutes at least 5 % of the entire tumor [4]. The current (2014) WHO classification of endometrial carcinomas [4] is summarized in Table 4.1. Other histological subtypes of endometrial carcinoma are rare, including neuroendocrine tumors and undifferentiated/dedifferentiated carcinomas.
Table 4.1
2014 World Health Organization classification of endometrial carcinomas
Histological type | Subtype | Histological variant | Histological variant |
|---|---|---|---|
Endometrioid carcinoma | Conventional | With squamous differentiation | With mucinous differentiation |
Secretory | |||
Villoglandular | |||
Microglandular | |||
Sertoliform | |||
Ciliated | |||
Mucinous carcinoma | |||
Serous carcinoma | Serous endometrial intraepithelial carcinoma (SEIC) | ||
Clear cell carcinoma | |||
Neuroendocrine tumors | Low grade | Carcinoid tumor | |
High grade | Small cell neuroendocrine carcinoma (SCNEC) | Large cell neuroendocrine carcinoma (LCNEC) | |
Undifferentiated carcinoma | Dedifferentiated | With trophoblastic differentiation | |
Mixed carcinomas |
The primary goal of intraoperative consultation for endometrial malignancy at the time of hysterectomy is to identify patients who are at high risk of pelvic and para-aortic lymph node involvement [5] and to avoid secondary staging surgery or suboptimal extended chemoradiation.
Indications for Frozen Section Diagnosis
Unexpected uterine mass
Clinical suspicion for endometrial malignancy without preoperative biopsy diagnosis
Confirming the diagnosis of carcinoma suspected on a prior biopsy
Assessing the overall FIGO grade of tumor
Determining the depth of myometrial invasion and extent of cervical involvement
Identifying a high-grade (type 2) carcinoma component (serous, clear cell, or malignant mixed Mullerian tumor)
In general, high-risk histological parameters include presence of type 2 carcinoma component, deep myometrial involvement, cervical stromal invasion, and adnexal metastasis. Although the grade of endometrial cancer is often available as a result of preoperative biopsy or curettage, endometrioid adenocarcinoma is not uncommonly heterogeneous with an admixed type 2 tumor component [6]. Therefore, gynecologic oncologists may request a frozen section even when the preoperative biopsy diagnosis is an endometrioid carcinoma.
Discrepancy between the endometrial biopsy/curettage and the staging hysterectomy is common with regard to tumor grade (31–54 % of cases), and the final grade is equally likely to be higher and lower than that of the prior biopsy [6, 7]. It is important to keep in mind that frozen section evaluation of endometrial cancer is not optimal, with significant disagreement reported between the frozen section and permanent diagnosis in terms of tumor grade, depth of myometrial invasion, cervical involvement, and lymph node metastasis. Such inherent limitation in predicting the final tumor grade and stage must be carefully considered by both the pathologist and gynecologic oncologist [8].
Specimen Processing for Intraoperative Consultation
Careful gross examination of the anterior and posterior endo-myometrium is important to identify the presence of invasive carcinoma.
For grossly identifiable lesions, one or two full-thickness sections of uterine wall should be submitted to evaluate the maximum depth of myometrial penetration. Sections of anterior and posterior cervix contiguous to the lower uterine segment should be submitted to assess cervical involvement.
If there is no grossly identifiable tumor or the preoperative biopsy showed atypical complex hyperplasia, representative anterior and posterior endo-myometrial sections should be submitted for frozen section evaluation.
Careful gross examination of adnexa is necessary, and frozen section evaluation of any suspicious lesions should be performed if endometrial malignancy is found.
Tumorlike Conditions
Endometrial Polyp [9–11]
Endometrial polyp is found in about 24 % of women in the general population and is a rather common finding in patients receiving tamoxifen treatment for breast cancer.
It may be sessile or pedunculated, single or multiple with size ranging from less than 1 cm to filling up the entire endometrial cavity (Fig. 4.1). Tamoxifen-related endometrial polyps tend to be large, multiple, and fibrotic grossly.
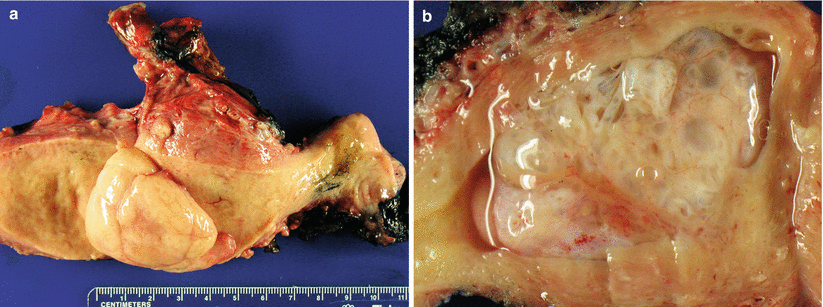
Fig. 4.1
Endometrial polyp. Pedunculated (a) or sessile (b) polypoid lesions that may fill the entire endometrial cavity
Polypoid configuration, fibrotic stroma, thick-walled vasculature, crowded, and/or irregular endometrial glands that may be proliferative, atrophic, or even secretory (Fig. 4.2).
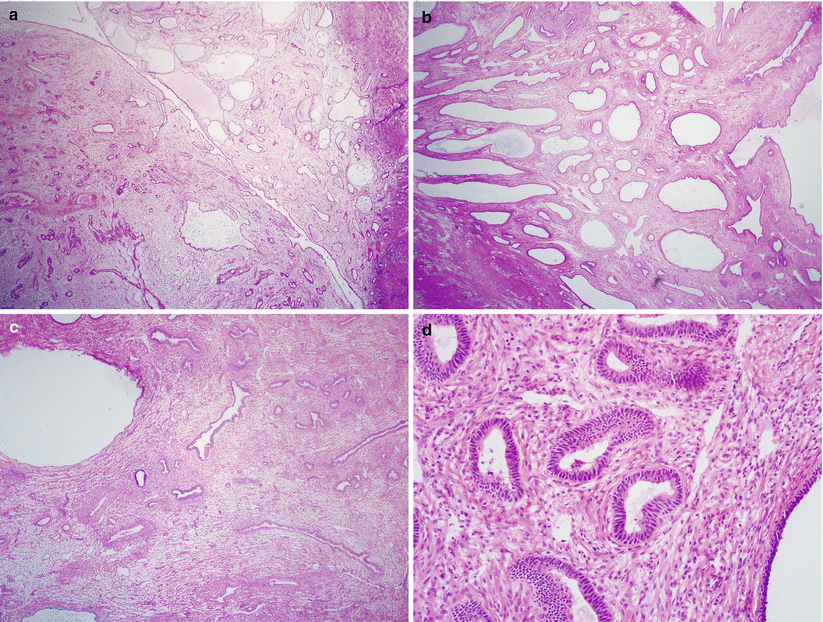
Fig. 4.2
Endometrial polyp. Note the polypoid configuration, fibrotic stroma, thick-walled vasculature, and irregular endometrial glands (a–c) and absence of cytological atypia (d)
Endometrial polyps in postmenopausal patients often have a more prominent fibrotic stoma and less glandular component. Metaplastic changes of the glandular epithelium, particularly endocervical-type mucinous metaplasia are common.
Endometrial polyps may be involved by preneoplastic conditions including complex mucinous changes, complex papillary proliferation, atypical hyperplasia (>10 % of cases), and early adenocarcinoma (2–3 % of cases).
Differential diagnosis
Polypoid adenomyoma
Atypical polypoid adenomyoma
Adenosarcoma
Diagnostic pitfalls/key intraoperative consultation issues
Endometrial polyp is separated from polypoid adenomyoma and atypical polypoid adenomyoma by lack of a significant smooth muscle component.
Endometrial polyp is separated from adenosarcoma by absence of periglandular stromal cell condensation, cytological atypia, and stromal cell mitotic activity.
In postmenopausal patients, careful screening for incidental minute foci of serous endometrial intraepithelial carcinoma (SEIC) within an endometrial polyp is important to ensure appropriate staging surgery at the time of frozen section examination.
Metaplastic and Reactive Changes [12–14]
Various endometrial metaplastic or reactive changes are frequently associated with or mimic neoplastic lesions of the endometrium.
Different types of metaplasias often coexist within the same specimen.
Eosinophilic and papillary syncytial metaplasias are often associated with endometrial breakdown, particularly abnormal bleeding.
Squamous metaplasia (often forming squamous morules) can be seen in benign endometrium, endometrioid adenocarcinoma, and its precursor—atypical endometrial hyperplasia.
Tubal or ciliated metaplasia has tubal-type epithelium and is often found in normal proliferative endometrium or in isolated glands of atrophic endometrium.
Clear cell metaplasia involves endometrial glands and shows clear cytoplasm containing glycogen.
Secretory changes can be superimposed on either hyperplasia or carcinoma with columnar cells having sub- or supranuclear vacuoles.
Mucinous changes, including papillary mucinous proliferation, are almost always of the endocervical type and can be seen in benign (endometrial polyp), premalignant, and malignant conditions.
Diagnostic pitfalls/key intraoperative consultation issues
Recognition of metaplastic changes on frozen section at the time of hysterectomy relies on lack of invasion, absence of significant cytological atypia, and minimal mitotic activity.
Mucinous changes with or without cytological atypia should prompt additional sampling to rule out a well-differentiated endometrioid carcinoma [15]. Highly complex mucinous proliferation with cribriform and branching villous epithelium and cytological atypia has a high frequency of associated carcinoma. Additional sections of the endometrium may be necessary to rule out invasive carcinoma at the time of intraoperative consultation.
Arias-Stella Reaction [16, 17]
Seen in patients with concurrent gestation, trophoblastic disease, or high-dose progestin use.
It may mimic tubulocystic histology of clear cell carcinoma by its glandular irregularity, cellular budding, striking nuclear atypicality, hobnailing, and clear cytoplasm.
Differential diagnosis
Clear cell carcinoma
Diagnostic pitfalls/key intraoperative consultation issues
Aria-Stella reaction is separated from clear cell carcinoma by its overall normal endometrial glandular distribution, absence of mitotic activity, and degenerative nuclear chromatin pattern.
Inquiry about concurrent gestational status or history of hormone therapy at the time of intraoperative consultation is helpful to confirm the diagnosis.
Endometrial Hyperplasia [18–20]
Clinical features
Perimenopausal patients often with obesity, polycystic ovary syndrome, and diabetes.
Atypical hyperplasia is generally considered a precursor lesion to endometrioid adenocarcinoma.
Gross pathology
Gross appearance is variable ranging from no obvious lesion to diffuse or polypoid endometrial thickening (Fig. 4.3).
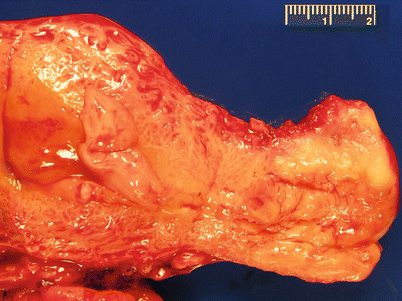
Fig. 4.3
Endometrial hyperplasia. Note the areas of marked endometrial thickening
Microscopic features
Hyperplasia without atypia (Fig. 4.4)
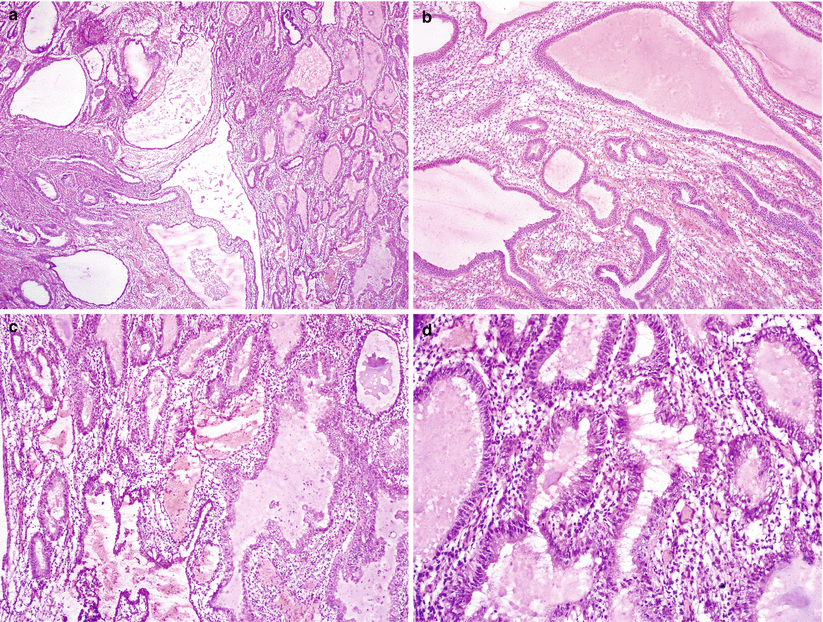
Fig. 4.4
Hyperplasia without atypia (simple hyperplasia without atypia). Diffuse proliferation of both glandular and endometrial stromal components with abnormal glandular architecture including cystic pouching and branching and absence of nuclear atypia (a–d)
Diffuse glandular proliferation with abnormal glandular architecture—irregular shapes (cystic, pouching, and branching glands) and variable sizes.
Either abundant (simple hyperplasia) or diminished stroma resulting in glandular crowding and back-to-back glandular configurations (complex hyperplasia).
Columnar or pseudostratified glandular cells with variable mitotic activity.
Lesser degree of proliferative process may be designated as disordered proliferative endometrium, showing isolated glandular abnormalities scattered within otherwise normal proliferative endometrium.
Atypical hyperplasia (Fig. 4.5)
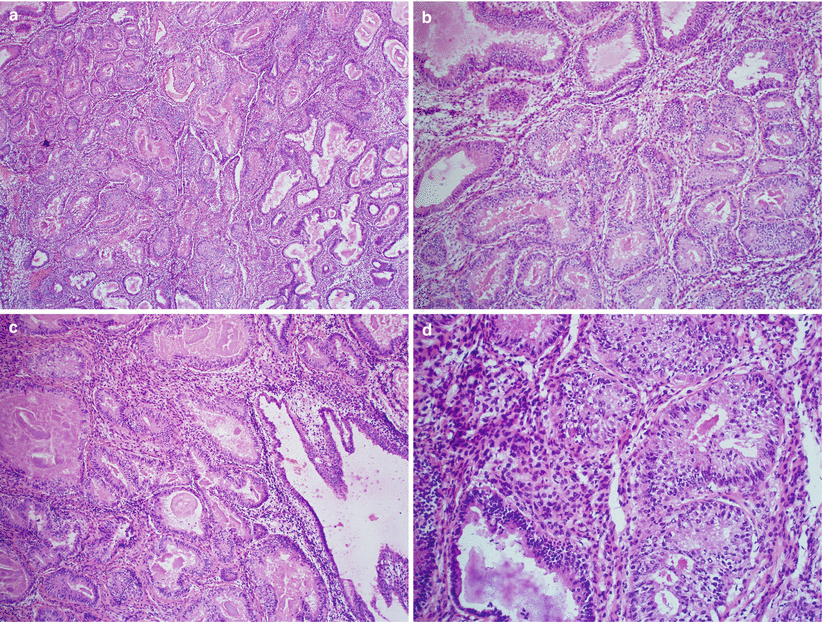
Fig. 4.5
Atypical hyperplasia (complex atypical hyperplasia). Note the glandular crowding (a, b) and the presence of cytological atypia including cytoplasmic eosinophilia, loss of nuclear polarity, nuclear enlargement, and rounding (c, d)
Hyperplasia with cytological atypia.
Diagnostic features of cytological atypia include:
Cytoplasmic eosinophilia
Loss of nuclear polarity
Nuclear enlargement, rounding, and variation in size
Thickening of the nuclear membrane and chromatin clumping
Nuclear atypia should involve a significant number or clusters of glandular cells.
Comparison with adjacent background normal, non-metaplastic endometrial glands is helpful to identify nuclear atypia.
Metaplastic changes—particularly mucinous metaplasia—frequently involve endometrial hyperplasia (Fig. 4.6).
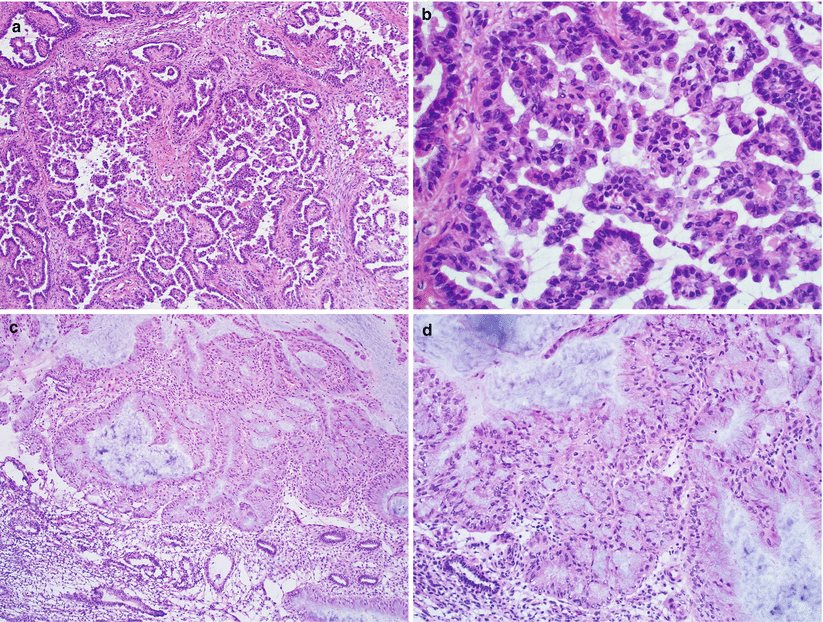
Fig. 4.6
Complex mucinous changes of the endometrium. Note the mucinous epithelium with a complex papillary configuration (a, b) or complex glandular patterns with mild nuclear atypia (c, d)
Differential diagnosis
Grade 1 endometrioid adenocarcinoma (see below)
Various endometrial epithelial metaplasias
Irregular endometrial glands at the basalis
Highly compact secretory endometrium
Endometrial polyp
Atypical polypoid adenomyoma
Diagnostic pitfalls/key intraoperative consultation issues
The single most important issue at the time of intraoperative consultation is to rule out carcinoma.
Grossly the most suspicious areas should be sampled for frozen sections.
Separation of atypical from non-atypical hyperplasia may be difficult but is not crucial at the time of frozen section evaluation.
Up to 40 % of hysterectomy specimens of patients with a biopsy diagnosis of atypical hyperplasia harbor grade 1 endometrioid carcinoma [21].
When atypical hyperplasia is diagnosed on frozen sections without definite evidence of carcinoma (Fig. 4.7), the entire endometrium needs to be submitted later for permanent microscopic examination. The surgeon should be informed of the possibility of endometrial carcinoma on permanent sections.
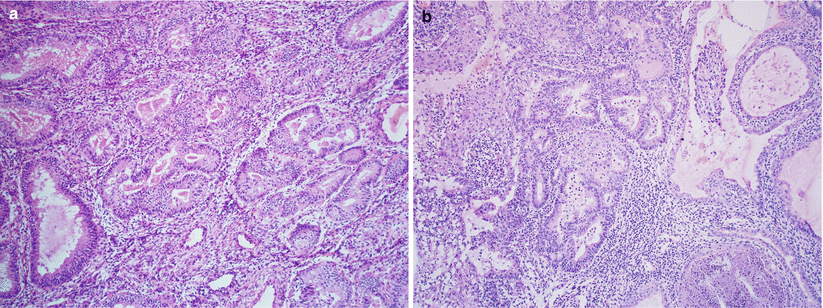
Fig. 4.7
Atypical hyperplasia bordering on well-differentiated endometrioid carcinoma. Note the foci of markedly atypical endometrial glands with intraglandular crowding and cribriform architecture (a, b)
Endometrioid Adenocarcinoma [4, 22]
Clinical features
In its pure form, the tumor represents 60 % of all uterine cancers and 20 % are mixed with other types of carcinomatous components.
Most cases occur in postmenopausal patients and present with vaginal bleeding.
Most patients have unopposed estrogen stimulation, due to obesity, chronic anovulation (e.g., polycystic ovary syndrome in a young patient), estrogen-producing ovarian tumors, estrogen replacement therapy, and breast cancer patients receiving tamoxifen treatment.
Lynch syndrome is a genetic predisposing factor for endometrial carcinomas, particularly endometrioid type.
Often preceded by atypical endometrial hyperplasia.
Gross pathology (Fig. 4.8)
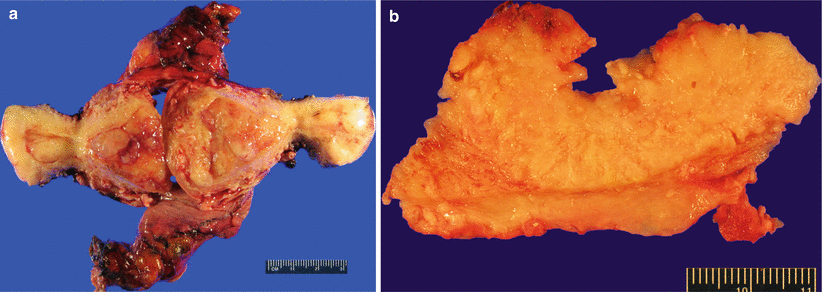
Fig. 4.8
Endometrioid adenocarcinoma. Multiple polypoid mass lesions involving the endometrium (a) and myometrium (b)
Single to multiple discrete polypoid masses or diffuse, exophytic endometrial growth.
Occasional cases may have an endophytic/infiltrative growth pattern with no obvious gross lesion.
Necrosis and hemorrhage are common.
Microscopic features
Confluent proliferation with glandular, villoglandular, or cribriform patterns (Fig. 4.9).
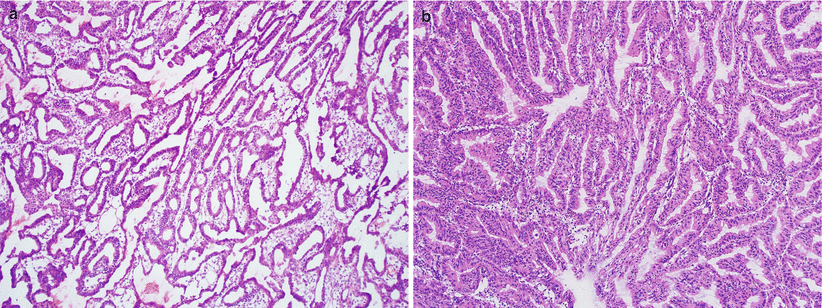
Fig. 4.9
Well-differentiated endometrioid adenocarcinoma. Note the presence of confluent, open glandular proliferation without a solid growth pattern (a, b)
Stratified glandular epithelium consisting of columnar tumor cells sharing a common apical cytoplasmic border, imparting on a smoothly contoured luminal surface (Fig. 4.10).
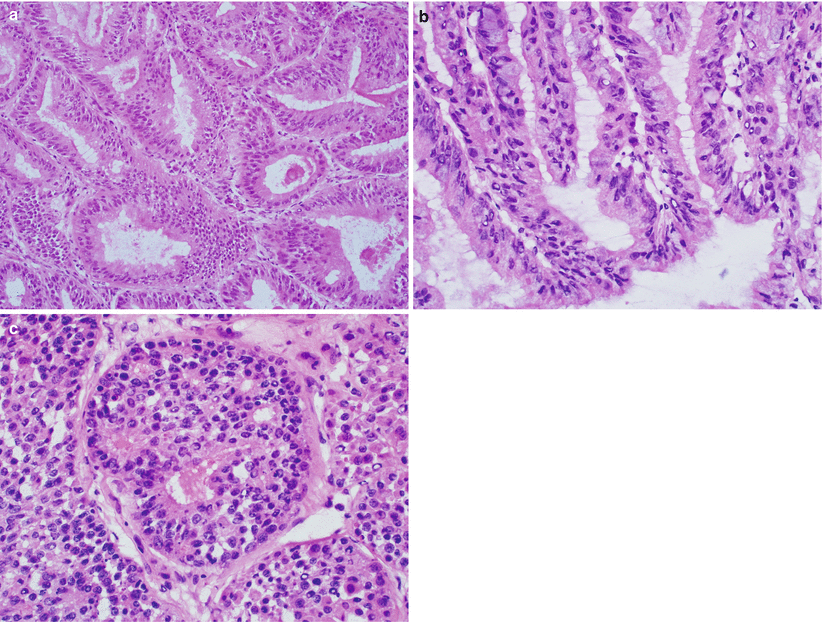
Fig. 4.10
Glandular features of endometrioid adenocarcinoma. Note the stratified glandular epithelium with columnar cells sharing a common apical cytoplasmic border, resulting in a smoothly contoured luminal surface (a–c)
Tumor cells have variable cytological atypia and mitotic activity.
Ciliated tumor cells are common.
Focal mucinous differentiation (<50 % of the entire tumor) is not an infrequent finding.
Frequent squamous differentiation, including mature (squamous morules, papillary surface squamous differentiation to overt keratinization) and immature types (squamous metaplasia to squamous carcinomatous changes).
Eosinophilic papillary syncytial metaplasia, luminal necrotic debris, and stromal foamy macrophages are also common.
Percentage of solid glandular growth determines the histological grade (FIGO grade) [23]: 5 % or less solid glandular component in FIGO grade 1, >5–50 % in grade 2, and >50 % in grade 3 tumors. Low FIGO grade tumor is upgraded by 1 grade when nuclear grade 3 involves more than 50 % of the entire tumor [24] (Figs. 4.11 and 4.12).
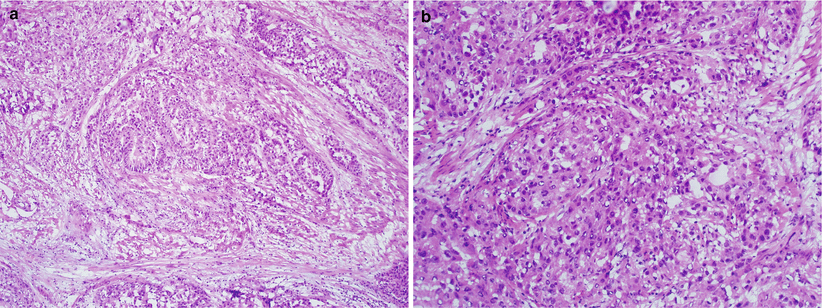
Fig. 4.11
FIGO grade 2 endometrioid adenocarcinoma. Note the presence of focal solid growth pattern (a, b)

Fig. 4.12
FIGO grade 3 endometrioid adenocarcinoma. Note the presence of predominant solid growth pattern (a–d) and marked nuclear atypia (c, d)
Variants of endometrioid adenocarcinomas [25–32].
Secretory carcinoma: endometrioid adenocarcinoma with a glandular growth pattern and tumor cells containing uniform subnuclear and/or supranuclear vacuolization.
Ciliated cell carcinoma: glandular growth pattern with extensive ciliated epithelial cells involving at least 75 % of the entire lesion.
Villoglandular variant: slender, delicate papillary fronds with thin, minimal stroma (Fig. 4.13).
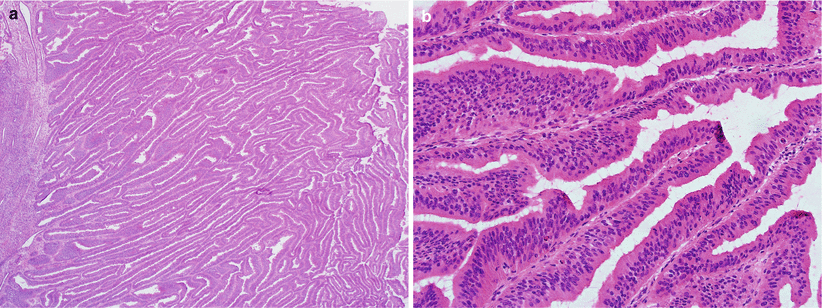
Fig. 4.13
Villoglandular endometrioid adenocarcinoma. Note the slender, delicate papillary fronds with thin or minimal stroma (a, b) and mild nuclear atypia (b)
Non-villous papillary endometrioid carcinoma: non-villous branching and budding papillae simulating serous carcinoma on low magnification, but the tumor cells are columnar with low nuclear grade (Fig. 4.14).
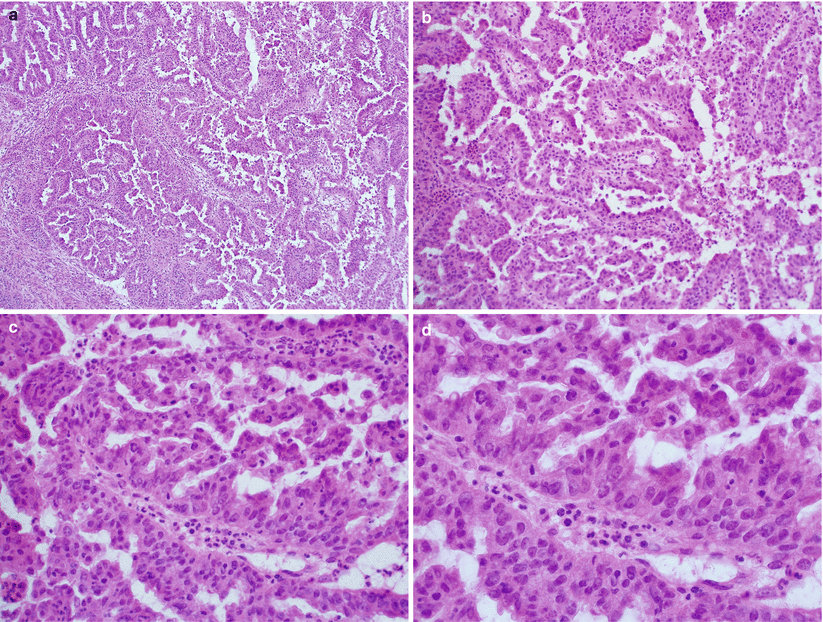
Fig. 4.14
Non-villous papillary endometrioid adenocarcinoma. Note the presence of non-villous papillae with short branching and budding, simulating serous carcinoma at low magnification (a, b). However, low nuclear grade with minimal mitotic activity is seen at higher magnification (c, d)
Microglandular endometrioid adenocarcinoma (Fig. 4.15): often mixed with conventional endometrioid adenocarcinoma with mucinous differentiation; areas of the tumor show microglandular growth pattern simulating endocervical microglandular hyperplasia.
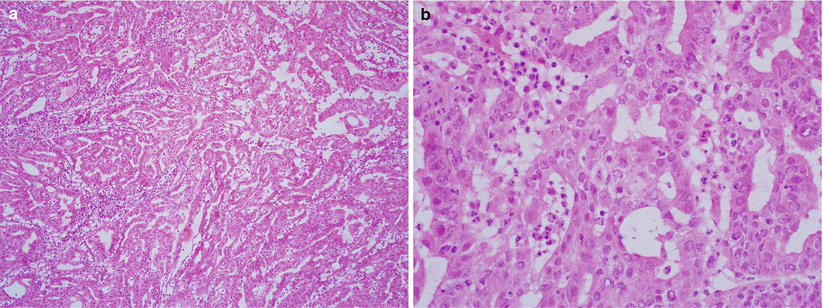
Fig. 4.15
Microglandular endometrioid adenocarcinoma. Note the well-differentiated endometrioid adenocarcinoma with microglandular growth pattern and inflammatory cells, simulating endocervical microglandular hyperplasia (a, b)
Sertoliform endometrioid adenocarcinoma: rare, well-differentiated endometrioid carcinoma showing focal or predominant cords to small, hollow tubular glands simulating Sertoli cell tumor of the ovary. The diagnosis relies on finding areas merging with conventional endometrioid carcinoma. Absence of endometrial stromal tumor component separates this variant from endometrial stromal tumor with sex-cord differentiation and uterine tumor resembling ovarian sex-cord tumor.
Differential diagnosis
Atypical complex hyperplasia
Serous carcinoma versus villoglandular, and non-villous papillary endometrioid adenocarcinoma
Clear cell carcinoma versus secretory endometrioid adenocarcinoma and endometrioid adenocarcinoma with squamous differentiation/clear cytoplasmic changes
Endocervical adenocarcinoma involving endometrium
Metastatic carcinomas from other sites (commonly breast and gastrointestinal origins)
Diagnostic pitfalls/key intraoperative consultation issues
Get Clinical Tree app for offline access
Separation of endometrial atypical hyperplasia from invasive carcinoma can be difficult, particularly at the time of frozen section evaluation. Thefollowing findings are indicative of carcinoma [18, 27] (see Fig. 4.7).
Desmoplastic stroma with fibroblasts or myofibroblasts rigidly existing between the atypical glands.
Stromal space replaced by aggregates of foamy macrophages or eosinophilic collagen.
Expansile glands with confluent, cribriforming pattern or extensive papillation.
When borderline histological findings are present and no gross invasion is found, frozen section interpretation as “markedly atypical hyperplasia, bordering on well-differentiated endometrioid carcinoma” may be communicated.
Lymphovascular invasion: true lymphovascular invasion should be carefully separated from mechanically displaced tumor cells, which is increasingly common in hysterectomy specimens obtained by robotic surgery. True lymphovascular invasion in the absence of myoinvasion by a low-grade endometrioid adenocarcinoma is very uncommon.
Cervical involvement (Fig. 4.16):
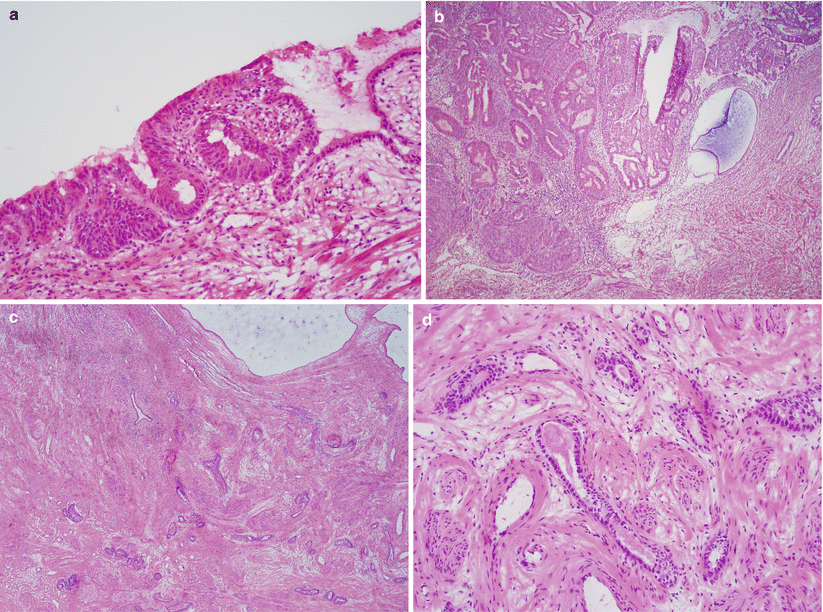
Fig. 4.16
Cervical involvement by endometrioid adenocarcinoma. The tumor may involve the endocervical mucosa (a) and stroma (b) and may assume deceptively benign-appearing tubular or cystic glandular patterns (c, d). Note the deep, haphazard infiltration of cervical stroma (c) and proximity to large-caliber vessels (d)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


