30
Endocrine Pancreas and Pharmacotherapy of Diabetes Mellitus and Hypoglycemia
This chapter will be most useful after having a basic understanding of the material in Chapter 43, Endocrine Pancreas and Pharmacotherapy of Diabetes Mellitus and Hypoglycemia in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. In addition to the material presented here, the 12th Edition includes:
• A detailed discussion of the regulation of blood glucose
• A description of pancreatic islet cell physiology and the synthesis and processing of insulin
• A discussion of the signaling pathway that is activated by insulin resulting in its effects on target cells
• A description of the pathogenesis of type 1 and type 2 diabetes
• Drugs that are in development for type 2 diabetes
• Table 43-5 Properties of Insulin Preparations
LEARNING OBJECTIVES
 Understand the mechanisms of action of insulin and the oral antidiabetic drugs.
Understand the mechanisms of action of insulin and the oral antidiabetic drugs.
 Describe the components for management of the diabetic patient including the goals of therapy.
Describe the components for management of the diabetic patient including the goals of therapy.
 Describe the pharmacotherapeutic options for the treatment of patients with type 1 or type 2 diabetes.
Describe the pharmacotherapeutic options for the treatment of patients with type 1 or type 2 diabetes.
 Learn about the adverse effects of insulin and the oral antidiabetic drugs.
Learn about the adverse effects of insulin and the oral antidiabetic drugs.
 Understand the treatment of hypoglycemia.
Understand the treatment of hypoglycemia.
DRUGS INCLUDED IN THIS CHAPTER
Acarbose (PRECOSE, others)
Alogliptin (NESINA)
Bromocriptine (CYCLOSET, not yet available in the United States for treatment of diabetes)
Colesevelam (WELCHOL)
Diazoxide (PROGLYCEM)
Exenatide (BYETTA)
Gliclazide (DIAMICRON, others, unavailable in the United States)
Glimepiride (AMARYL)
Glipizide (GLUCOTROL, others)
Glucagon
Glyburide (Glibenclamide; MICRONASE, DIABETA, others)
Insulin aspart (NOVOLOG)
Insulin determir (LEVEMIR)
Insulin glargine (LANTUS)
Insulin glulisine (APIDRA)
Insulin lispro (HUMALOG)
Insulin protamine hagedorn (NPH, insulin isophane)
Liraglutide (VICTOZA)
Metformin (GLUCOPHAGE, others)
Miglitol (GLYSET)
Nateglinide (STARLIX)
Pioglitazone (ACTOS)
Pramlintide (SYMLIN)
Repaglinide (PRANDIN)
Rosiglitazone (AVANDIA)
Saxagliptin (ONGLYZA)
Sitagliptin (JANIVIA)
Vidagliptin (GALVUS, ZOMELIS, JAIRA, not available in the United States)
Voglibose (VOGLIB, not available in the United States)
MECHANISMS OF ACTION INSULIN AND ORAL ANTIDIABETIC DRUGS
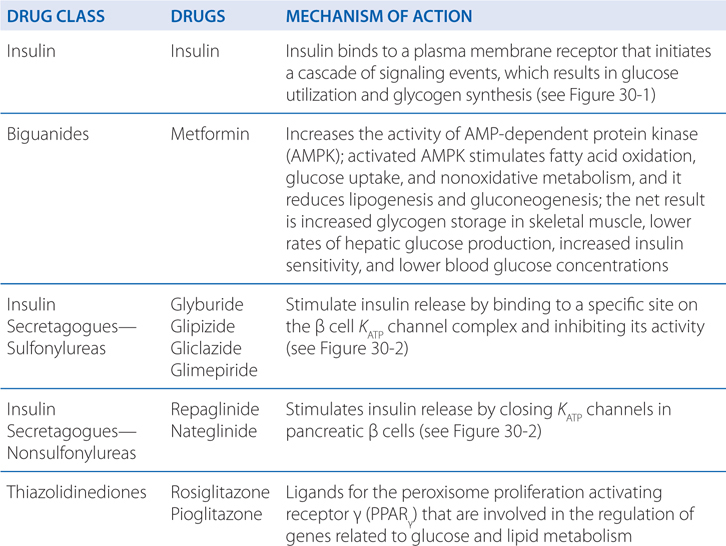
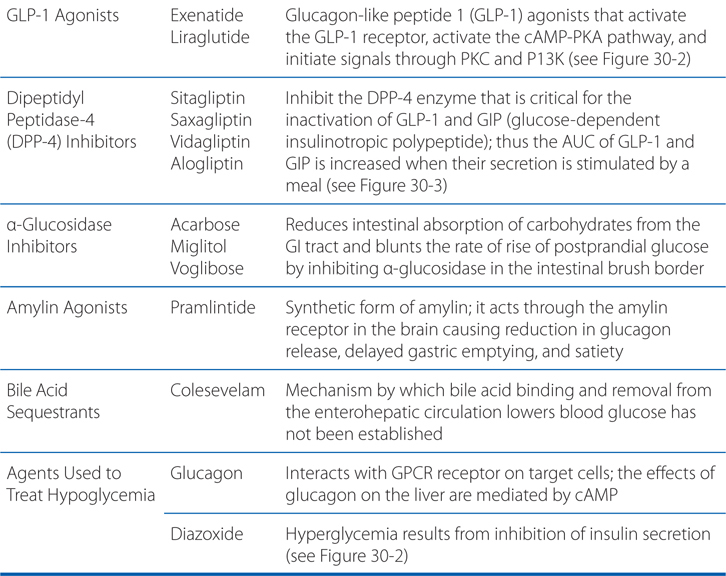
FIGURE 30-1 Pathways of insulin signaling. The binding of insulin to its plasma membrane receptor activates a cascade of downstream signaling events. Insulin binding activates the intrinsic tyrosine kinase activity of the receptor dimer, resulting in the tyrosine phosphorylation (Y-P) of the receptor’s β subunits and a small number of specific substrates (light blue shapes): the insulin receptor substrate (IRS) proteins, Gab-1 and SHC; within the membrane, a caveolar pool of insulin receptor phosphorylates caveolin (Cav), APS, and Cbl. These tyrosine-phosphorylated proteins interact with signaling cascades via SH2 and SH3 domains to mediate the effects of insulin, with specific effects resulting from each pathway. In target tissues such as skeletal muscle and adipocytes, a key event is the translocation of the Glut4 glucose transporter from intracellular vesicles to the plasma membrane; this translocation is stimulated by both the caveolar and noncaveolar pathways. In the noncaveolar pathway, the activation of PI3K is crucial, and PKB/Akt (anchored at the membrane by PIP3) and/or an atypical form of PKC is involved. In the caveolar pathway, caveolar protein flotillin localizes the signaling complex to the caveola; the signaling pathway involves a series of SH2 domain interactions that add the adaptor protein CrkII, the guanine nucleotide exchange protein C3G, and small GTP-binding protein, TC10. The pathways are inactivated by specific phosphoprotein phosphatases (eg, PTB1B). In addition to the actions shown, insulin also stimulates the plasma membrane Na+,K+-ATPase by a mechanism that is still being elucidated; the result is an increase in pump activity and a net accumulation of K+ in the cell. Abbreviations: aPKC, atypical isoform of protein kinase C; APS, adaptor protein with PH and SH2 domains; CAP, Cbl associated protein; CrkII, chicken tumor virus regulator of kinase II; GLUT4, glucose transporter 4; Gab-1, Grb-2 associated binder; MAP kinase, mitogen-activated protein kinase; PDK, phosphoinositide-dependent kinase; PI3 kinase, phosphatidylinositol-3-kinase; PIP3, phosphatidylinositol trisphosphate; PKB, protein kinase B (also called Akt); Y, tyrosine residue; Y-P, phosphorylated tyrosine residue.
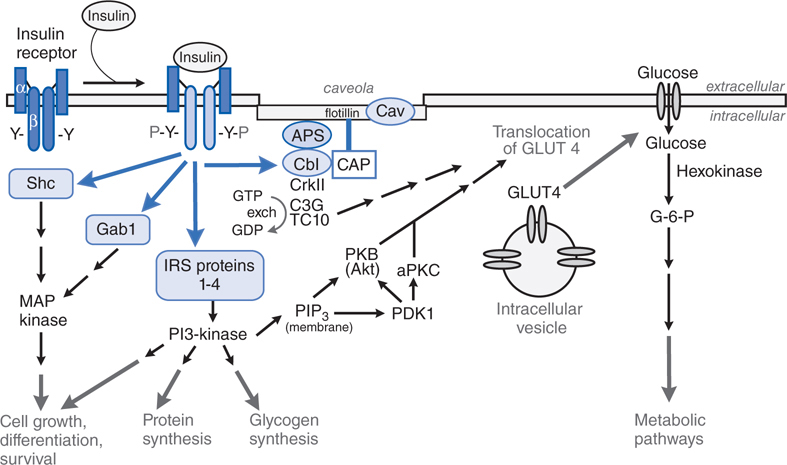
FIGURE 30-2 Regulation of insulin secretion from a pancreatic β cell. The pancreatic β cell in a resting state (fasting blood glucose) is hyperpolarized. Glucose, entering via GLUT transporters (primarily GLUT1 in humans, GLUT2 in rodents), is metabolized and elevates cellular ATP, which inhibits K+ entry through the KATP channel; the decreased K+ conductance results in depolarization, leading to Ca2+-dependent exocytosis of stored insulin. The KATP channel, actually a hetero-octamer composed of SUR1 and Kir 6.2 subunits, is the site of action of several classes of drugs: ATP binds to and inhibits Kir6.2; sulfonylureas and meglitinides bind to and inhibit SUR1; all 3 agents thereby promote insulin secretion. Diazoxide and ADP-Mg2+ (low ATP) bind to and activate SUR1, thereby inhibiting insulin secretion. Incretins enhance insulin secretion.
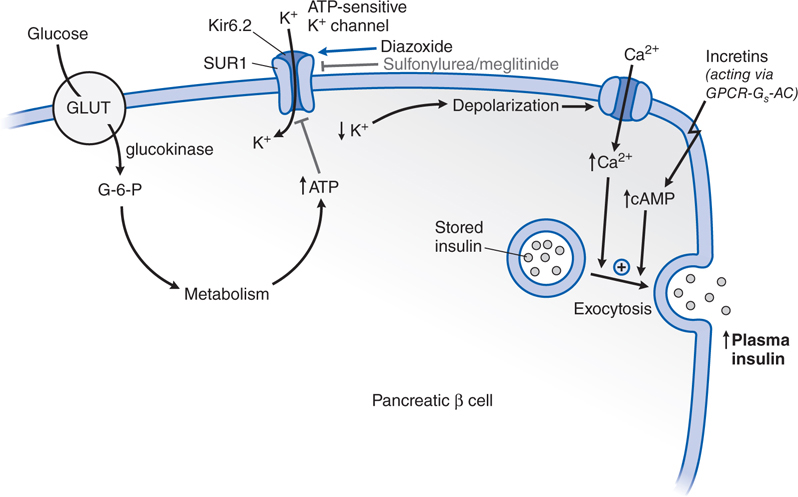
FIGURE 30-3 Pharmacological effects of DDP-4 inhibition. DPP-4, an ectoenzyme located on the luminal side of capillary endothelial cells metabolizes the incretins, glucagon-like peptide 1 (GLP-1), and glucose-dependent insulinotropic polypeptide (GIP), by removing the 2 N-terminal amino acids. The target for DPP-4 cleavage is a proline or alanine residue in the second position of the primary peptide sequence. The truncated metabolites GLP-1 [9-36] and GIP [3-42] are the major forms of the incretins in plasma and are inactive as insulin secretagogues. Treatment with a DPP-4 inhibitor increases the concentrations of intact GLP-1 and GIP.
A 10-year-old girl is diagnosed with type 1 diabetes. She will start on insulin therapy.
a. How is the diagnosis of type 1 diabetes made?
The diagnosis of diabetes mellitus is currently based on the correlation of diabetes-specific complications with a particular level of glycemia, that is, the level of glycemia at which a diabetes-specific complication like retinopathy begins to appear. The criterion for the diagnosis of diabetes is shown in Table 30-1.
TABLE 30-1 Criteria for the Diagnosis of Diabetes
• Symptoms of diabetes plus random blood glucose concentration ≥11.1 mM (200 mg/dL)a or
• Fasting plasma glucose ≥7.0 mM (126 mg/dL)b or
• Two-hour plasma glucose ≥11.1 mM (200 mg/dL) during an oral glucose tolerance testc
• HbA1c ≥6.5%
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree