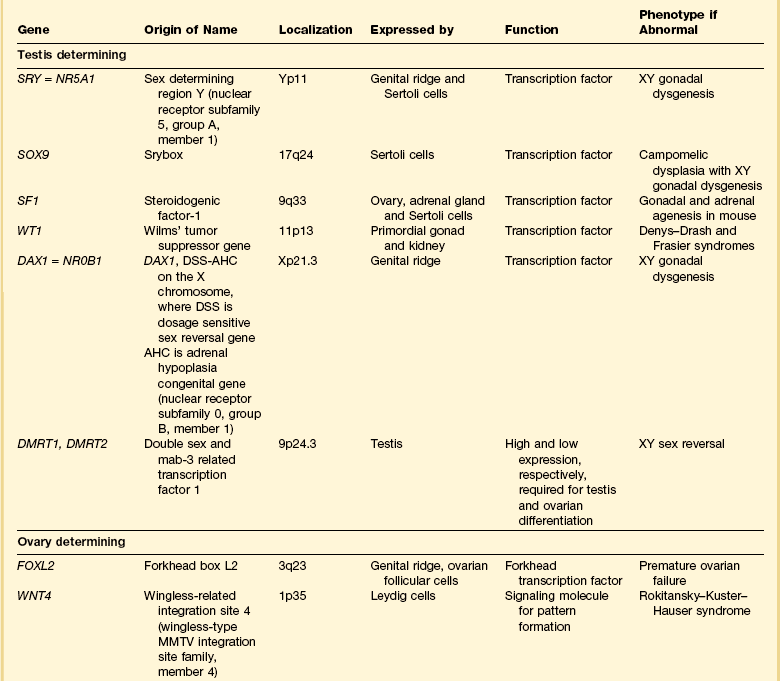
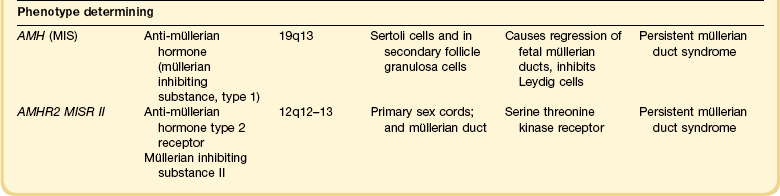
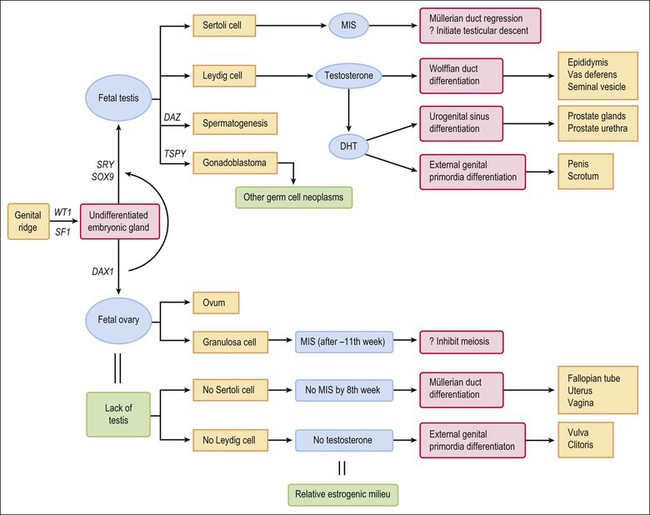
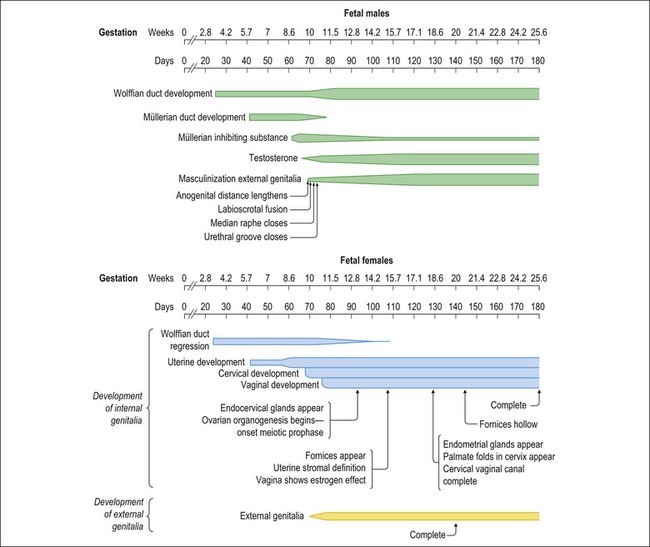
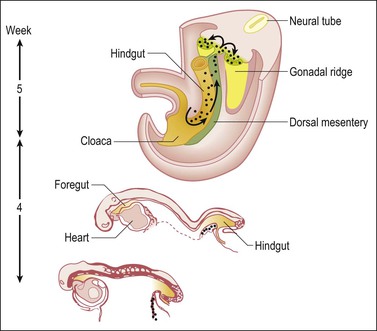
1 Understanding normal development of the embryonic genital tract gives insight into many disorders encountered in the female. These can range from relatively simple arrests of development or malformation (described by organ) to more complex abnormalities of sexual development that result from dysembryogenesis (see Chapter 2), and, in some cases, to help understand the origin of some tumors, particularly sex cord–stromal and germ cell tumors of the ovary. Most early insights have come from understanding human mutations. During more recent years, targeted mutations using mouse models have disclosed key roles for genes that had not been anticipated previously. Many regulators of gonadal development are receptors, signal transduction elements, transcription factors, extracellular ligands, and even intracellular signaling pathways mediating downstream transcriptional responses. Recently published references1–7 provide extensive reviews and, to some degree, competing theories. Most of the female genital tract is of mesodermal origin. Germ cells are of endodermal origin. The vulva and the epithelial lining of the vagina are ectoderm. The chronology and sequence of events that underlie the development of the female genital tract are summarized in Figures 1.1 and 1.2, and in Table 1.1. Table 1.2 lists specific genes involved in the initial steps of sexual development. Table 1.1 Synopsis of Stages of Normal Embryologic Development Figure 1.2 Normal sexual development. Embryologic development is determined by several factors, all of which are time specific during embryogenesis. In the broadest view, sex determination takes place in three sequential steps.8 The first is chromosomal sex determination, which occurs as a result of fertilization. Gonadal sex determination, the second critical event, results when the potential gonads actually transform into ovaries or testes in accord with the available chromosomal information. Third, the secondary sex characteristics develop along female or male lines as determined by the preponderant estrogenic or androgenic hormonal milieu present systemically. Sexual identity, not to be discussed here, includes a person’s sense of self (gender identity) and his/her attraction to others. In humans and other mammals, the karyotype ‘XY’ genetically defines the sex as male, whereas ‘XX’ defines the female sex. Sex is determined by the presence or absence of a signal from the substance initially called the testis determining factor and now recognized as the gene called SRY (Sex determining Region Y) in the human and SRY in the mouse. The gene is found on the Y chromosome. Testes are formed if this gene is expressed by the embryo before the urogenital ridge differentiates. Further male development occurs under the influence of hormones secreted later by the testes. Without SRY, the gonads differentiate as ovaries and the embryo develops as a female. The timely expression of SRY is critical to the development of male sex. In its absence, the embryo develops a female phenotype, regardless of genetic sex. Although for years believed to occur by default, a gene has been identified in women (R-spondin1, RSPO1)9 critical for development of the ovary through signaling pathways.10 The SRY gene is located in the region just central to the pseudoautosomal pairing region at the distal end of the short arm of the Y chromosome.11 The pseudoautosomal pairing region is named for the two limited regions at the distal ends of the short and long arms of the Y chromosome where sequence identity with the X chromosome permits pairing and recombination during male meiosis.12 The gene, which has a strongly conserved motif,13 encodes for a DNA binding protein, which is the binding activity product (transcriptional switch) that orchestrates the action of other genes. It does so by initiating a cascade of gene expression that regulates the development of the testis, not all of which are known or understood. Several lines of evidence support this thesis. These include that the: • SRY gene is absent from the normal X chromosome and somatic chromosomes • SRY gene is present on the X chromosome of ‘sex-reversed’ XX human males • Homologous gene in the mouse is initially expressed just before sexual differentiation begins • SRY gene acts in the absence of germ cells • SRY gene in the chromosomally female embryo causes it to develop as a male.14 Rare examples have also been identified where single base pair point mutations in the SRY gene or in promoter regions essential for gene expression render an XY patient as a phenotypic female with a streak gonad.15 Early on, SRY initiates induction of somatic cell migration from the mesonephros into the gonad16 and induces indifferent cells in the genital ridge to differentiate into Sertoli cells. This is the first type of cell required to form in the embryonic testis.17 With monoclonal antibodies, an SRY protein has been found in the nuclei of Sertoli cells and germ cells.18 Several other genes are thought to be important in Sertoli cell function. SOX9, which is named for it being an SRY-related high motility gene box group, in the mouse is active in pre-Sertoli cells19 and results in XY human females when mutations inactivate the gene. SF1, which SOX9 helps regulate, is another important gene that is an orphan nuclear receptor expressed in the gonadal ridge in precursor cells of Sertoli and stromal cells. It appears as a master regulator of the reproductive system because it regulates the expression of numerous genes required for gland development and hormone synthesis.20–22 Mutations in this gene in humans have been responsible for adrenal insufficiency associated with gonadal dysgenesis.23 The gene DAX1, which is required at several points in embryonic testis development,24 also plays a key role in sex determination. Overexpression causes varying degrees of gonadal dysgenesis, and at high doses in the mouse, male-to-female sex reversal occurs. Additional references8,12,22,25 describe in greater detail the genes involved in the rapidly evolving science of male sex determination. During the development of both male and female human embryos, but before the gonads develop, the primordial germ cells migrate from the yolk sac to the urogenital ridges via the caudal part of the hindgut approximately 3 weeks after fertilization (Figure 1.3). The yolk sac, which is of considerable size (Figure 1.4), is easily recognized by its reactivity to α-fetoprotein. This migratory event is independent of eventual sex. The germ cells are large and prominent, and have clear cytoplasm and vesicular nuclei. Once they synthesize glycogen and alkaline phosphatase, they are easily identified histochemically by their demonstration of placental-like alkaline phosphatase (PLAP) and CD117 (c-kit). Figure 1.3 Migration of germ cells in the human embryo. At the end of the third week, epiblast-derived cells present in the yolk sac near the allantoic base have differentiated into primordial germ cells, the latter having migrated by the fifth week along the dorsal mesentery of the hindgut to the gonadal ridges.
Embryology
Introduction
Postovulatory Week
Crown–Rump at Beginning of Week
Sequence of Events (Top to Bottom) Within Stated Week
1 week
0.2 mm
Uterine implantation (0.2 mm).
2 weeks
1.5 mm
Primitive pit forms. No somites yet.
3 weeks
2.0 mm
1–3 somites.
Pronephric tubules form; pronephric (mesonephric) duct arises and grows caudad as solid cord (2.5 mm).
Primordial germ cells first discernible in yolk sac near caudal part of embryo (2–3 mm).
Pronephros degenerated, but mesonephric duct reaches cloaca (3–5 mm).
Primordial germ cells discernible in hindgut (3–5 mm).
Primordial germ cells discernible in mesonephric ridges (5 mm).
4 weeks
7 mm
Primordial germ cells discernible in gonadal ridge, which itself at this time is a thin mesodermal proliferation (7 mm).
Cloaca divides into rectum and urogenital sinus.
Müllerian ducts appear as funnel-shaped opening of celomic epithelium.
5 weeks
12 mm
Indifferent gonad bulges into celom (12 mm).
Primitive sex cords appear (16–17 mm).
6 weeks
18 mm
Müllerian ducts about half distance to urogenital sinus.
Testis anatomically distinct with seminiferous tubules and capsule (tunica albuginea).
7 weeks
23 mm
Ovaries initially identified by absence of distinct seminiferous tubules (23 mm).
Müllerian ducts elongate and near urogenital sinus (23 mm).
Müllerian ducts in apposition; sinusal tubercle appears (23–28 mm).
Müllerian ducts lose sensitivity to MIS at 25 mm, but regression effect not observed until 31–35 mm.
8 weeks
29 mm
Müllerian ducts fuse and in contact with urogenital sinus (29 mm).
9 weeks
43 mm
Müllerian duct regression in response to MIS completed by 43–55 mm.
Testes and ovaries acquire capacity to secrete characteristic hormones at same stage of development; testosterone coincides with histologic development of Leydig cells and immediately precedes virilization of genital tract; ovary not yet differentiated; rate-limiting step is appearance of 3-α-hydroxysteroid dehydrogenase, which is 50-fold more abundant in testis than in ovary; ovary converts testosterone to estradiol, which testis cannot do; later regulation shifted to pituitary–placenta gonadotropins where testosterone → estradiol controlled by conversion of cholesterol to pregnenolone.
Müllerian ducts completely fused (entire septum gone); caudal aspect proliferates; epithelium lining canal stratifies (2–3 cells layers thick).
10 weeks
60 mm
Anogenital distance lengthens.
Testosterone synthesis sufficient to induce development of mesonephric duct into definitive structures (epididymis, vas deferens, and seminal vesicle). Subsequently, testosterone converted peripherally into 5-α-dihydrotestosterone, which causes the following transformations:
Urogenital sinus → prostate
Genital tubercle → glans penis
Genital folds → penis (only 3.5 mm long)
Genital swelling → scrotum
Fusion of labioscrotal folds.
Closure of median raphe.
Closure of urethral groove.
Phallus in both sexes 3 mm long; thereafter grows in males 0.72 mm/week and females 0.20 mm/week.
Mesonephric ducts regress if not stimulated by testosterone.
Vaginal plate first seen distinctly (complete at 140 mm; week 17). Initially, upper uterovaginal canal is large and oval in cross section, mostly lined by pseudostratified columnar epithelium. Extensive growth begins caudally; cells stratify.
Uterovaginal canal occluded caudally, progresses cranially.
11 weeks
71 mm
Primordial follicles appear.
Seminal vesicles develop.
Testis at inguinal ring.
Extensive uterovaginal growth continues caudally.
12 weeks
93 mm
Cervical glands appear; wavy, but undifferentiated.
Vaginal rudiment approaches vestibule.
True ovarian organogenesis begins with onset of meiotic prophase.
13 weeks
105 mm
Male urethral organogenesis complete.
14 weeks
116 mm
Primary folds of mucosa give uterine lumen W-shaped appearance on cross section.
Vaginal rudiment reaches level of vestibular glands; uterovaginal canal (15 mm total length) divisible into vagina (one-half), cervix (one-third), and corpus (one-sixth); boundaries ill-defined.
Isthmus readily distinguishable.
Stromal layers of uterus begin definition.
Solid epithelial anlage of anterior and posterior fornices appear.
Vagina begins to show slight estrogen effect.
15 weeks
130 mm
Fallopian tube begins active growth phase, begins to coil.
Vaginal plate completed; lower end reaches vestibule; upper end extends into endocervical canal.
Female urogenital sinus becomes shallow vestibule.
Primary follicles of ovary appear.
16 weeks
142 mm
Vaginal plate longest and begins to canalize.
Corpus glands appear as slight outpouchings.
17 weeks
153 mm
Palmate folds of cervix appear (forerunner adult cervix).
Mucoid development of cervix begins.
Smooth muscle of uterus appears.
Estrogen effect apparent throughout vagina.
Cavitation of vaginal canal completed.
18 weeks
164 mm
Fornices hollow.
19 weeks
177 mm
20 weeks
186 mm
Dramatic increase in growth and coiling of fallopian tube (about 3 mm/week to week 34).
21 weeks
197 mm
22 weeks
208 mm
Differentiation of muscular layer of uterus complete.
Fundus well marked; uterus assumes adult form.
Graafian follicles appear.
24 weeks
230 mm
26 weeks
250 mm
28 weeks
270 mm
30 weeks
290 mm
34 weeks
328 mm
38 weeks
362 mm
Birth.
Gonadal Development
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree