a pleural, pericardial, or peritoneal fluid is of capital importance for the patient and the attending physician or surgeon. Although, in many such instances, a fatal outcome of the disease may be anticipated, some tumors offer a much better prognosis than others. For example, metastatic mammary carcinoma may be controlled, often for a period of many years, by various forms of therapy. In some other tumors, long-term remissions or even cures can be achieved. Malignant lymphomas, some testicular tumors, and some malignant tumors of childhood, such as neuroblastoma or embryonal rhabdomyosarcoma, may respond to energetic therapeutic measures. Therefore, the responsibility of the pathologist is two-fold:
To identify cancer cells accurately
To identify tumor type and, if possible, the site of primary origin
The body fluids are a natural tissue culture medium, wherein mesothelial and tumor cells may proliferate free of the boundaries imposed upon them by the framework of organs and tissues (also see comments in Chap. 25). It is known to all students of in vitro tissue culture that morphologic identification of benign versus malignant cultured cells may be fraught with considerable difficulty. Similarly, the characteristic features of human cancer cells in fluids may undergo substantial modifications. For example, abnormal cell shapes that often help in the identification of exfoliated or aspirated cancer cells may no longer be present in fluids, wherein the cancer cells may assume a neutral, spherical appearance. Nuclear features often seen in cancer, such as hyperchromasia, may also be absent or attenuated.
Proliferating mesothelial cells may conceal the presence of tumor cells or may mimic cancer cells by forming complex clusters or displaying alarming nuclear features, such as the presence of nucleoli (see Chap. 25). Rarely, clusters of macrophages (histiocytes) may mimic cancer.
Large or very large. The cells are significantly larger than normal mesothelial cells. Some mesotheliomas, metastatic carcinomas of various types, malignant melanomas and sarcomas belong in this group. When combined with abnormal nuclear features, described below, the identification of such tumors is easy (see Figs. 26-36B, 26-40D, 26-57).
Small. The tumors are made up of cells much smaller than mesothelial cells. Most malignant lymphomas, many of the malignant tumors of childhood (neuroblastoma, Wilms’ tumor) and certain carcinomas (small-cell carcinoma of the breast, oat cell carcinoma) belong to this group of tumors. Close attention must be paid to nuclear features and interrelationship of cells for accurate identification (see Figs. 26-28, 26-30, 26-35).
Medium-sized. The diagnostic problem usually occurs with cells of medium size which are approximately of the same size as mesothelial cells. A variety of carcinomas of mammary, lung, gastric, pancreatic, or prostatic origin may have this presentation (see Fig. 26-26). This is perhaps the most difficult group of tumors to identify and the most important source of diagnostic error.
in identifying cancer. Thus, the presence of bizarre or spindly cells (see Figs. 26-19, 26-20, and 26-57), columnar cells or cells resembling bronchial lining cells is usually associated with cancer (see Figs. 26-34, 26-41). Exceptions may occur, for example in the sediment in rheumatoid arthritis wherein spindly epithelioid cells may be observed (see Chap. 25). Very rarely, benign fibroblasts, derived from connective tissue supporting the mesothelium, may also be observed in such fluids.
TABLE 26-1 CELL FEATURES USEFUL IN THE IDENTIFICATION OF CANCER CELLS IN EFFUSIONS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(see Fig. 26-41). Such cells must be differentiated from “signet ring” macrophages that have normal, small nuclei (see Chap. 25). Intracytoplasmic glandular inclusions (“target cells” or “bull’s eye cells”) are observed mainly in mammary carcinoma (see Fig. 26-28D) but may sometimes occur in other tumors as well (Kumar et al, 2000).
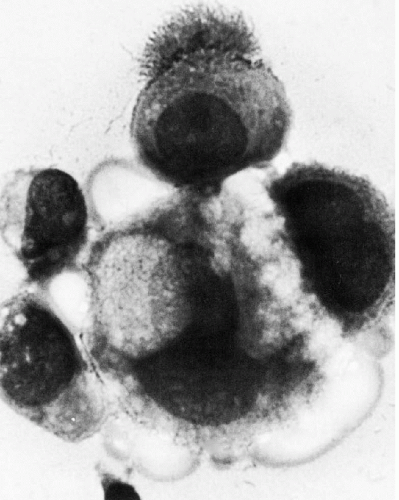
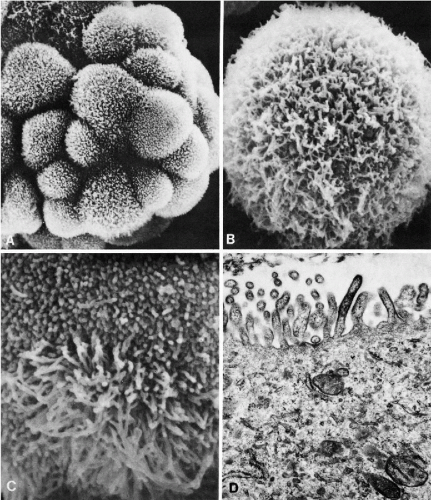
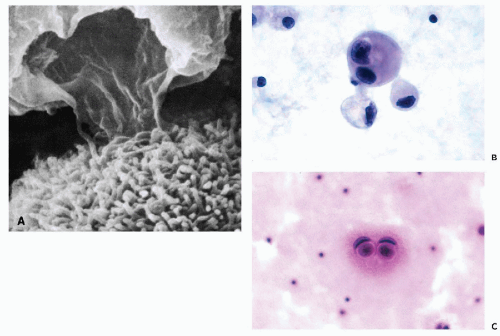
these commonly encountered cell clusters are actually elaborate, organized 3-dimensional structures. Spriggs divided the structures into three groups: papillary, tubulopapillary, and acinar (Fig. 26-5). On cross-section, the cells composing the clusters surround a lumen, form cell junctions, and often contain central deposits of collagen as supporting structures. The papillary and tubulopapillary clusters are provided with microvilli on their outer surfaces, whereas the acinar structures contain microvilli on the surface facing the lumen. Thus, the cell aggregates, far from being haphazard accumulations of epithelial cells, are, in fact, highly organized structures formed by benign or malignant cells, growing freely in effusions.
 Figure 26-3 Microvilli on the surface of cancer cells. A. Alcohol fixation. B. Air-dried smear (oil immersion). The microvilli are better seen in the air-dried material. |
with smooth borders. Occasionally, on closer scrutiny, an irregular nuclear outline may be observed and is of diagnostic assistance, particularly in malignant lymphomas, in the form of indentations or protrusions of the nuclear membrane. Another nuclear feature observed in malignant effusions, particularly in malignant lymphomas, is massive nuclear breakdown (apoptosis or karyorrhexis) that is virtually never seen in benign fluids (see Figs. 26-46, 26-47).
chromatin granules, suggestive of a mitotic prophase (Fig. 26-6D). These findings are uncommon in benign cells and should also be considered as a possible presumptive evidence of a malignant tumor.
immortality on cancer cells. Telomerase activity can be demonstrated by molecular biologic techniques or by immunochemistry using an in situ fluorescent assay and a telomere repeat amplification protocol (TRAP) (Ohyashiki et al, 1997). The test results in nuclear fluorescence in cancer cells whereas, in benign cells, the fluorescence is limited to the cytoplasm. Dejmek et al (2001) adopted this technique to cells in 16 effusions and claimed that the test was specific for cancer cells. Similar data were previously provided for cancer cells in the respiratory tract (Dejmek et al, 2000) and in urinary sediment (Ohayashiki et al, 1998). However, Braunschweig et al (2001) denied any diagnostic value to the TRAP reaction because of frequent false-positive and false-negative results.
significant clinical value. This information may help in the determination of the organ of origin of the tumor and provide guidance to optimal treatment. The best chances of identification of tumor type occur when cancer cells form multicellular structures akin to those observed in tissues or if fragments of tumor can be recognized in cell blocks. Other identification options are based on cell relationships and identification of cell products. The three most common types of tumors encountered in effusions are: adenocarcinomas, poorly differentiated carcinomas of various origins, and small cell tumors. Less often, keratinizing squamous carcinoma may also be recognized. Many of the features of malignant cells described above may serve to identify tumor types.
TABLE 26-2 MONOCLONAL ANTIBODIES USED ON EFFUSIONS | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
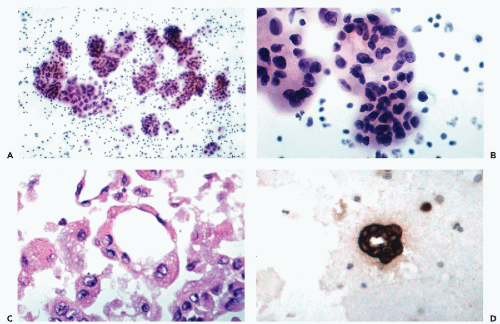
The tumor cells form gland-like or tubular structures with a central lumen (see Fig. 26-4).
Tumor cells forming multilayered, spherical or oval cell clusters, suggestive of papillary growth, also known as “spheroids” or “hollow spheres” (see Fig. 26-4A,B). In paraffin-embedded cell blocks, cross-sections of such clusters usually reveal glandular features of adenocarcinoma (see Fig. 26-4C,D).
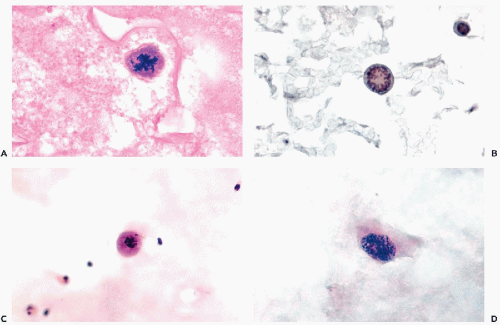
Single cells of adenocarcinoma may be recognized if they are of columnar configuration because such cell types practically never occur in other tumor types.
Signet ring cancer cells, with an abnormal nucleus displaced to the periphery by a large mucus vacuole (see Fig. 26-41
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree