Echinococcal Cyst—Open Approach
Miroslav Milicevic
The Liver Echinococcal Cyst
Definition, Short History, Epidemiology, Significance, and the Surgical Problem
Echinococcosis (echinococcal cyst [EC], hydatidosis, hydatid disease) is a cyclozoonosis caused by the larval (metacestode) stages of cestodes (flat worms) belonging to the genus Echinococcus and the family Taeniidae. The biologic characteristics of the parasite enable its survival in nature. The disease exists in two forms: the larval stage (metacestode) and the adult stage (tenia). The parasites are perpetuated in life cycles with carnivores (dogs and wild canine) as definitive hosts. Humans are the accidental intermediate host (dead end) and animals (herbivores and omnivores) are both intermediate and definitive hosts.
The disease has been known since earliest times. It was known to Hippocrates, who speaks of “livers full of water.” The word echinococcus is of Greek origin and means “hedgehog berry.” Hydatid is also of Greek origin (hudatid, hudatis) and means a “watery vesicle.” The word hydatid also originates from the modern Latin word hydatis meaning a “drop of water.” Therefore, hydatid and cyst have the same meaning and the expression “hydatid cyst” (HC) is a pleonasm. The use of the term hydatid cyst in contemporary medicine is widespread and acceptable. The first North American case was observed in 1808 and published in 1822. The true nature of the disease was not known until the second half of the nineteenth century.
Echinococcosis is widespread and occurs in all continents, including circumpolar, temperate, subtropical, and tropical zones. It is not confined to sheep-raising communities. The varying prevalence and distribution is influenced by agricultural, educational, economical, medical, and cultural factors. In endemic areas, the annual incidence of cystic echinococcosis ranges from <1 to 200 per 100,000 inhabitants. Human cystic echinococcosis remains highly endemic in pastoral communities, particularly in regions of South America (Argentina, Uruguay, Chile), the Mediterranean littoral (Spain, France, Italy), Eastern Europe, the Near and Middle East (Turkey), East Africa (Maghreb countries), Central Asia, China, and Russia. Increasing migration, growing incidence of world trade and travel, high mobility of troops, and an increasing number of refugees make hydatidosis a global problem. In endemic areas hydatidosis remains a major health and socioeconomic problem. The estimated minimum global human burden of CE averages 285,000 disability-adjusted life years (DALYs) or an annual loss of U.S. $194,000,000. The mortality rate from cystic echinococcosis is about 2% to 4% but it may increase considerably if medical treatment and care are inadequate. Only infection with the metacestode is of clinical relevance.
Although echinococcosis is uncommon in the resident population of industrially developed countries, it is prevalent enough to be seen by most general surgeons in its simple or its complicated form. Because it is a surprise finding, even in tertiary centers, it is overtreated more often than not.
The treatment of hydatid disease has been the responsibility of surgeons for many years, and research into its treatment also seems to be the responsibility of surgeons. There is no other parasitic disease for which the primary treatment is surgical. Because there is no effective treatment of hydatid disease today, either surgical or medical, the best surgical technique is not known. Surgeons will be happy when a proper medical treatment is found and they are needed only for the complications.
A surgeon managing a patient with a HC should understand both the parasitology and pathology of the parasite and be fully aware of treatment limitations and possible complications. The dilemma of whether to perform tissue-sparing, conservative, or radical resectional surgery for hydatid disease is an artificial issue. The operative procedure should be tailored to every individual patient, taking into consideration many factors including the local expertise and facilities, prevalence of the disease, socioeconomic factors, and the age and condition of the patient.
This chapter presents the basics of this disease and it is intended to help the surgeon manage patients with HCs.
Current Concepts in the Diagnosis and Management of Ec
The previous recommendations of the World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) for the treatment of human echinococcosis were published in 2001. These recommendations have significantly influenced the way cystic echinococcosis was managed in all continents. Ten years later, after several expert meetings of the WHO-IWGE, a consensus was reached in 2009 about the management of cystic echinococcosis. The consensus has been reached on an image-based, stage-specific approach, which helps in choosing one of the following treatment options: (a) surgery, (b) percutaneous treatment (PT), (c) chemotherapy, and (d) watchful waiting. An image-based classification system differentiating the cyst stage has been reinforced to help in clinical decision making. Unfortunately, the panel of experts also concluded that the majority of published papers about the management of cystic echinococcosis were not consistent and well structured, so the quality of evidence and strength of recommendation is low. Since cystic echinococcosis is mostly treated in developing countries with limited resources, choosing the best treatment option must take into consideration the socioeconomic conditions, the available facilities, and the expertise of the attending surgeon. Nevertheless, surgery remains the most effective treatment. It is frequently technically demanding and risky and postoperative complications can be life threatening.
This chapter is based on these new concepts.
The Liver Hydatid Cyst
Parasitology
The genetic and phenotypic variations of E. granulosus have been extensively studied. The dog is the most common definitive host and the majority of cases in humans are caused by the sheep strain. The adult tenia lives adhered to the small intestine of the dog (definitive host). It measures from 3 to 6 mm in length, and has three or four proglottids, of which the last one is larger and gravid. The gravid proglottid containing between 200 and 800 ova is shed approximately every 15 days and the ova are expelled in the feces. When the microscopic eggs are ingested by an intermediate host the life cycle is perpetuated. The intermediate hosts are Bovidae.
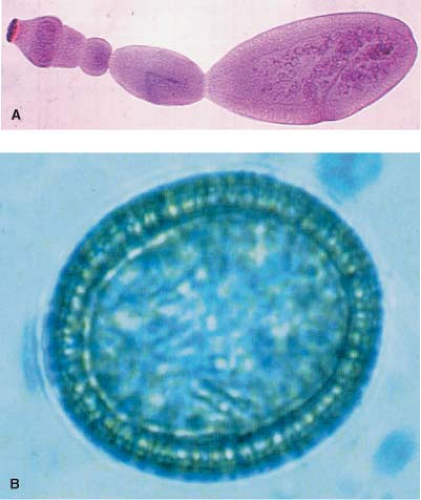
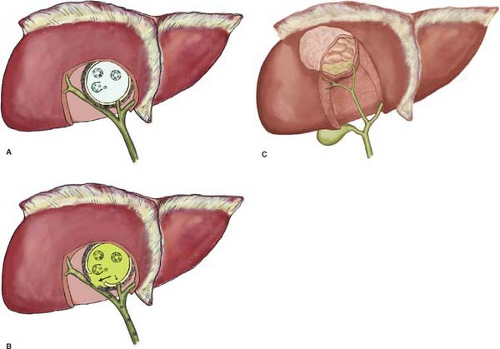
There are three known forms of echinococcosis in humans: (a) cystic echinococcosis (hydatid disease) caused by E. granulosus, (b) alveolar echinococcosis (alveolar hydatid disease) caused by E. multilocularis, and (c) polycystic echinococcosis caused by E. vogeli or E. oligarthus (?). Although E. granulosus and E. multilocularis occur simultaneously in large endemic areas, mixed infections of cystic echinococcosis are extremely rare. This chapter deals with cystic echinococcosis (hydatid disease) caused by E. granulosus only (Fig. 1).
Humans are an accidental, intermediate host and become infected when they accidentally ingest eggs of the tapeworm. This can happen directly, by contact with dogs, or indirectly by food, water, and contaminated objects. Direct contact during childhood is an important route. The eggs hatch in the duodenum, and the released oncosphere penetrates the mucosa and reaches a blood vessel. The bloodstream can carry the oncosphere to any part of the body, but it most frequently settles in the liver and lungs. Once settled, the parasite develops its larval stage, the HC, the clinical presentation of E. granulosus. The cyst is a chronic, well-localized, and adapted space-occupying affliction that is not affected by the functional status of the host. The protoscolex can multiply asexually ad infinitum in the intermediate host as long as the host is alive. Because the parasite is always confined within the HC, a vital hydatid cyst has the tendency to slowly enlarge and interact with the host. Following a symptom-free period of varying length, depending on the site and number of cysts, life-threatening complications arise. The disease, benign in nature, can cause devastating damage to the liver and death to the patient.
This cycle with two hosts, one definitive and the other intermediate, is the sexual cycle and the resulting disease in humans or animals is “primary echinococcosis.” There is an asexual, “minor” cycle in which the new HCs develop from any vital element of the larval stage (e.g., protoscoleces, daughter cysts) of the parasite in the same intermediate host. This can occur subsequent to invasive and operative procedures, or following spontaneous or trauma-induced cyst rupture. This disease in humans is “secondary echinococcosis.”
Pathology
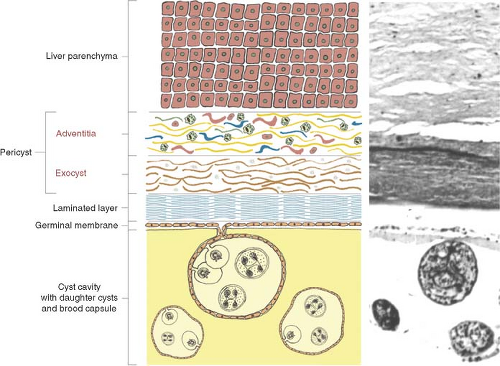
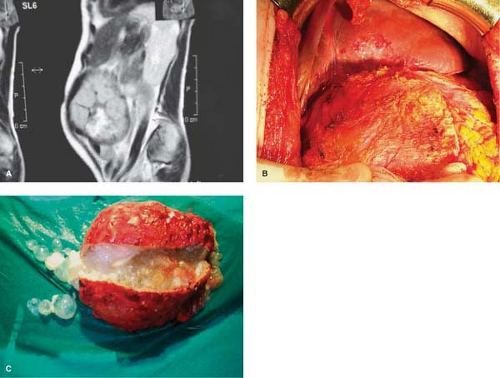
The cellular response of host tissue to the presence of the larva is an attempt to encase the parasite in fibrous tissue. The parasite reacts by forming around itself a spherical enclosure of inert protective chitinous material. If the parasite survives host defense mechanisms, in approximately 5 to 7 days after infestation, the larva (metacestode) develops into a 6- to 7-mm vesicula. This is the endocyst and it is made up of two distinct layers: (a) the inner germinative layer (live parasite, germinal membrane, proligerous layer) and (b) a protective outer laminated acellular layer (cuticle or laminated layer). The laminated membrane never grows thicker than 2 to 5 mm, regardless of cyst size. After 21 days, the cyst is visible to the naked eye. In 3 months it can measure 3 to 5 cm in diameter. Compression and interaction of the endocyst with the adjacent host’s tissue produces a fibrous layer called the ectocyst or pericyst. By 5 months the pericyst is formed and a typical mature HC is present.
The pericyst is an integral part of both the liver and the parasite, and it is difficult to remove it from the liver. The parasite (endocyst) is separated from the pericyst (ectocyst) by a small capillary space and it can easily be detached from it (Figs. 2 and 3). The pericyst is largely avascular but there are spaces within it that enclose blood vessels and small bile ducts. The structure of the pericyst seems to be more complex than previously described. According to Chinese authors it is the result of two different formative processes and it consists of two different layers. The inner layer of the pericyst (the frank “exocyst”) is the result of an inflammatory and an unusual granulomatous reaction aimed at walling of the parasite and resorbing the released antigen. The granulomatous layer increases in size with growth of the parasite; it frequently contains osteopontin (regulates macrophage accumulation and calcium deposition) and resembles the granulomatous reaction to other infective agents (e.g., TBC). The outer layer of the pericyst is called the “adventitial” layer and it is composed of compressed Glissonian biliovascular elements and tributaries of the hepatic veins. Separating the “ectocyst” from the “adventitial” layer is the basis of “subadventitial cystectomy” pioneered by Chinese authors. There is no cleavage plane between the liver parenchyma and the “adventitia” and it cannot be readily separated. The pericyst can be 1 to 1.3 cm thick and it is not present in pulmonary and brain ECs. Vessels and ducts in the “adventitia” remain patent for a long time even in large cysts (hypervascular rim on computerized tomography [CT] scan). Calcification impedes the passage of nutrients and oxygen into the cyst.
A liver HC is typically unilocular. A developing cyst usually survives, with a steady
increase in size of about 1 to 1.5 mm in diameter per month, depending on the site and adjacent structures. The hydatid fluid in a vital cyst is under pressure (30 to 70 cm H2O), and the volume of a human HC can be many liters.
increase in size of about 1 to 1.5 mm in diameter per month, depending on the site and adjacent structures. The hydatid fluid in a vital cyst is under pressure (30 to 70 cm H2O), and the volume of a human HC can be many liters.
The germinal membrane is of microscopic dimensions and is responsible for the production of the crystal-clear hydatid fluid, the ectocyst, brood capsules, scoleces, and the daughter cysts. The host and parasite in a HC are never physically connected. This means that a HC always separates from, or can be readily lifted out from within, a surgically exposed pericystic cavity (Fig. 3). When managing HCs, it must be assumed that any amount of hydatid fluid, no matter how clear, contains potentially infective protoscoleces.
Daughter cyst formation is considered a defense reaction and it is known as the process of endogenic vesiculation. Daughter cysts are true replicas of the mother cyst but without a pericyst. Daughter cysts produce multivesicular cysts that are more frequent in adults. The presence of daughter cysts is a problem for chemotherapy, protoscolicide activity, and the standard PAIR procedure (puncture–aspiration–injection–reaspiration). Ectogenic vesiculation of E. granulosus is infrequent. It occurs when there is a small rupture or defect in the laminated membrane and the germinal layer passes through and creates a “satellite” HC (Figs. 4 and 5).
Hydatid fluid is antigenic. This antigenicity is rarely of great clinical significance and the real incidence of allergic reactions in hydatidosis is not known. The incidence appears to be lower than previously described in literature. Allergic reactions range from skin rash to a frank anaphylactic reaction, usually following sudden rupture of the cyst. The antigenicity of hydatid fluid is the basis of serodiagnostics.
HCs in humans are generally long-standing and large, although not all grow to enormous proportions. Host defense mechanisms, injury, and the functional status of the pericyst are factors that determine cyst vitality, growth, and development. When a HC dies its wall can become calcified. Cyst vitality should not be estimated according to the calcification pattern. Dead cysts are generally no longer a threat to their host, but the issue of how to manage patients should take into consideration many factors.
The Uncomplicated Liver Hydatid Cyst
The clinical features of liver hydatid disease depend on the site, size, number, vitality, and stage of development of the cyst. Simple, uncomplicated liver HCs are usually asymptomatic or present with nonspecific symptoms. Complicated liver HCs cause specific symptoms. If a patient has multiple HCs in the liver, the cysts are different in size, and any one of them can be the cause of symptoms. Most frequently it is the complicated cyst that causes the symptoms and signs of the disease.
I base my experience of this topic on 1,168 consecutive patients operated for
liver hydatidosis at the First Surgical Clinic, University of Belgrade School of Medicine, during January 1963 to December 2009. Dominant symptoms and signs of the patients are presented in Tables 1 and 2. Only one in three symptomatic patients had moderate to severe pain. The majority of the patients (79.88%), had uncomplicated cysts.
liver hydatidosis at the First Surgical Clinic, University of Belgrade School of Medicine, during January 1963 to December 2009. Dominant symptoms and signs of the patients are presented in Tables 1 and 2. Only one in three symptomatic patients had moderate to severe pain. The majority of the patients (79.88%), had uncomplicated cysts.
Symptomless, unsuspected HCs can be detected during routine examination or at autopsy. Clinical latency, judging by the size of the HCs at operation, is an outstanding feature of the disease. Adults contracting the disease can rapidly develop severe clinical manifestations. Children in endemic areas with large cysts can progress to “hydatid cachexia” (Tables 1 and 2).
The Complicated Liver Hydatid Cyst
Suppuration and Secondary Bacterial Infection
Cyst leakage is a prerequisite for bacterial contamination, and the most frequent cause of infection is a cystobiliary communication (CBC). The clinical presentation is as a liver pyogenic abscess. An infected HC undergoes structural changes. The parasite dies.
Occasionally, the entire cyst content undergoes aseptic necrosis, the parasite dies, and the cyst is filled with amorphous, yellow debris that must be distinguished from the pus of secondary infection (e.g., positive Gram stain). Essentially, this is the gross appearance of a multivesicular cyst and not a suppurated cyst. The real incidence of bacterial infection is unknown because different diagnostic criteria are applied. The incidence in the literature ranges from 11.0% to 27.1%. It is 11.64% (136 patients) in my series.
Pressure Effects, Rupture, and Bile Duct Communications
A viable liver HC has the tendency to grow in the direction of least resistance. This accounts for its frequently irregular shape. In confined areas, such as the central nervous system, small cysts cause serious symptoms. In less restricted areas, the latency period is longer and the symptoms depend on the site and size of the cyst. Pressure effects appear sooner or later and symptoms result from direct pressure or distortion of neighboring structures or viscera.
An enlarging cyst causes compressive atrophy of surrounding hepatocytes and fibrosis, which can lead to compensatory hypertrophy of the remaining liver parenchyma. Large cysts can replace an entire liver lobe. Portal hypertension and a Budd–Chiari-like syndrome have been described. Another serious consequence of cyst enlargement is that it can rupture. Three types of cyst rupture have been addressed: (a) obscure, (b) free, and (c) communicant rupture.
Obscure (Internal) Rupture
Injury or penetration of bile into the space between the pericyst and the endocyst can cause rupture of the laminated membrane. The liberated protoscoleces occupy the available space and develop into hundreds of daughter cysts within the pericyst cavity. A typical univesicular cyst becomes multivesicular. When such a cyst is surgically entered, there is no laminated membrane and hundreds of daughter cysts, floating in a yellowish fluid and gelatin-like amorphous mass, crowd the interior of the pericyst. Not all multivesicular cysts have bile-stained fluid, and not all cysts with bile-stained fluid have active communications with the bile ducts. A multivesicular cyst with viable daughter cysts retains its high intracystic pressure, continues to enlarge, and can damage the host (Fig. 4).
The clinical significance of multivesicular cysts is that (a) the host is exposed to hydatid antigens in the hydatid fluid, (b) the cyst is bacteriologically sterile, (c) the cyst contents cannot be easily aspirated and need to be scooped out, (d) the cyst must be treated as viable and infective, and (e) bile-stained cyst contents mandate a meticulous search for CBC.
Table 1 Dominant Symptoms of Liver Hydatidosis | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
Table 2 Dominant Signs of Liver Hydatidosis | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
Free Rupture
In free rupture, the hydatid contents disseminate throughout the peritoneal or pleural cavity.
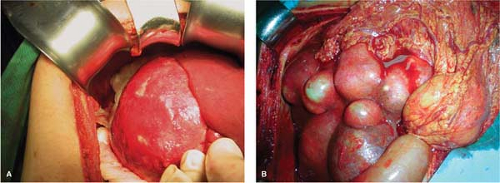
Intraperitoneal Rupture. A vital HC grows in the direction of least resistance, usually reaching the surface of the liver before it reaches enormous proportions. The superficial portion of the pericyst is stretched, thinned out, and the cyst becomes visible as an irregularly shaped, fibrous, whitish structure protruding from normal liver parenchyma. Cysts reaching the anterior and inferior part of the liver continue to grow, protruding into the abdominal cavity. Because of high intracystic pressure, both univesicular and multivesicular cysts can rupture.
There are several clinical presentations of intraperitoneal rupture: (a) In acute symptomatic rupture, peritoneal irritation and acute abdominal symptoms occur. This is an uncommon event. The incidence is about 1% to 4%. (b) In anaphylactic shock, rupture of the hepatic HC precipitates severe circulatory collapse, which may be fatal, and tends to mask the abdominal manifestations. (c) In silent rupture, the patient presents with disseminated
abdominal hydatidosis, unaware when the rupture occurred. (d) Herniation of the laminated membrane (“dumbbell hepatoperitoneal cyst”) occurs through the pericyst. The herniating membrane does not actually burst and therefore no spillage of hydatid debris occurs. The initial liver cyst remains small although the herniated, extrahepatic portion of the cyst can attain a volume of several liters. This condition mimics ascites, and attempts at percutaneous aspiration can lead to allergic manifestations. It is an infrequent condition (Fig. 6).
abdominal hydatidosis, unaware when the rupture occurred. (d) Herniation of the laminated membrane (“dumbbell hepatoperitoneal cyst”) occurs through the pericyst. The herniating membrane does not actually burst and therefore no spillage of hydatid debris occurs. The initial liver cyst remains small although the herniated, extrahepatic portion of the cyst can attain a volume of several liters. This condition mimics ascites, and attempts at percutaneous aspiration can lead to allergic manifestations. It is an infrequent condition (Fig. 6).
Intraperitoneal rupture is a life-threatening complication that results in “secondary echinococcosis.” Multiple cysts develop throughout the peritoneal cavity, causing intestinal obstruction, gross abdominal distention, ascites, and cachexia several years after the rupture. This is the secondary, smaller life cycle for the parasite, occurring only in the intermediate host. In endemic areas the sudden onset of bile-colored ascites in an otherwise healthy person, regardless of the fact that no hepatic cyst is visible, raises suspicion of intraperitoneal rupture.
Intrathoracic Rupture. An elevated hemidiaphragm and a sterile sympathetic pleural effusion can be the first signs of liver hydatid disease. Upward extension of a subdiaphragmatic cyst is usually asymptomatic, although it can cause dry cough, dyspnea, chest pain, and toxemia. The pleura and adherent basal lung segments often become inflamed and indurated. Frank intrapleural rupture with empyema (hydatopiothorax) is rare. A combination of infection and pressure can cause destruction of lung parenchyma, resulting in pneumonitis or lung abscess. A HC may erode into a bronchiole and the contents can be evacuated. Rupture into the lumen of a bronchiole may lead to the appearance of daughter cysts in the sputum. If the cyst is already communicating with the bile ducts, a bronchobiliary fistula will arise. Expectoration of bile-tinged sputum is a sign of bronchobiliary fistula. The incidence of diaphragmatic or transdiaphragmatic thoracic involvement by HCs in the dome of the liver ranges from 0.6% to 16%.
Communicant Rupture
HCs can rupture into physiologic channels (e.g., biliary, blood vessels) or adjacent organs (e.g., digestive tract) (Fig. 7).
Intrabiliary Rupture. Compression and displacement of biliary ducts is frequent. Enlargement of the HC stretches and compresses the bile duct, causing bile stasis. At the point of intimate contact, the cyst wall and bile duct gradually weaken. The increase of intraductal pressure facilitates occurrence of horizontal or longitudinal fissures in the duct wall. Bile escapes through this breach of the bile duct wall, decompressing the bile duct and accumulating at the external side of the laminated membrane (internal rupture). This “leak” causes changes in osmotic pressure and a further increase in intracystic pressure that facilitates rupture into the bile duct (external rupture). Because the pressure in the cyst greatly exceeds that in the bile duct, a cystobiliary fistula is frequent. The communication with the bile duct can be terminal or tangential. Peripheral cysts disrupt small-caliber ducts (usually end to
side) and central cysts communicate with a major segmental or sectoral duct (usually side to side). A single cyst can have several CBCs to the same or to different bile ducts.
side) and central cysts communicate with a major segmental or sectoral duct (usually side to side). A single cyst can have several CBCs to the same or to different bile ducts.
In silent rupture, bile leaks from eroded small ducts into the cyst, causing endogenic vesiculation, suppuration, and eventually death of the parasite. Such cysts are filled with bile-stained detritus, although no visible bile duct communications can be seen. Probably 80% to 90% of HC bile duct ruptures are of the silent type. This type of asymptomatic, silent rupture is known as a “minor communication” but, nevertheless, it can sometimes cause unexpected postoperative bile leakage.
A triad of symptoms characterizes “symptomatic” rupture into the bile ducts: (a) biliary colic, (b) partial intermittent or complete ductal obstruction with cholangitis and jaundice, and (c) germinative membranes in the feces. Rupture into a large bile duct may allow more or less complete emptying of the fluid and detritus and lead to spontaneous cure or cholestatic jaundice with recurrent cholangitis. Incomplete emptying and a persisting communication usually result in secondary infection. In endemic areas, probably 3% to 10% of cases of obstructive jaundice are caused by intrabiliary rupture of hepatic HCs. This type of rupture is also called a “major communication,” has a fistula orifice diameter of >5 mm, and communicates with a larger bile duct. This type of rupture is infrequent and has an incidence of 3% to 10%.
The rapid discharge of the cyst contents into a major bile duct or body cavity can lead to the sudden absorption of the hydatid antigen in a sensitized patient, resulting in anaphylaxis. More frequently, pruritus or urticarial rash is the major external manifestation. Episodes of asthma have been reported.
The incidence of rupture of the HC into the bile ducts is difficult to establish. The reported incidence ranges from 2.6% to 30.0%. This complication accounts for 60% of all the complications of hydatid disease and 20% of the postoperative morbidity. In my series, the incidence was 20.46%.
Rupture into Adjacent Organs. A HC can rupture into the digestive tract, causing hydatidemesis or hydatidenteria. Rupture into the urinary tract causes hydatiduria. Rupture of HC into the aorta, the inferior vena cava (VCI), the pericystic blood vessels, and the heart with embolism has been described.
Spontaneous Abortion
The parasite survives because of a complex interaction with the host through the pericyst. It is not known why some cysts occasionally stop developing and growing. Involution and death of the parasite are probably the result of malfunction of the pericyst or some other unknown noxious factor. Involution of the parasite is protracted, can probably last for years, and is accompanied with gross structural changes. The final phase is partial or total calcification of the pericyst.
Organ Imaging in Diagnosis and Treatment of Hydatid Disease
Roentgenographic Examination
The value of radiography in the diagnosis of uncomplicated liver HCs is very limited because the radiodensity of a noncalcified HC is the same as that of surrounding liver parenchyma. In endemic areas, elevation of the right hemidiaphragm in an otherwise
healthy, asymptomatic patient is highly indicative of liver hydatidosis. Sometimes a standard radiographic examination of the abdomen reveals streak-like or round calcification of a senile HC. Cysts in the lung and bone are more readily demonstrated. Angiography and scintigraphy are of historic significance only.
healthy, asymptomatic patient is highly indicative of liver hydatidosis. Sometimes a standard radiographic examination of the abdomen reveals streak-like or round calcification of a senile HC. Cysts in the lung and bone are more readily demonstrated. Angiography and scintigraphy are of historic significance only.
Ultrasound Imaging
Ultrasonography
Ultrasound imaging (US) has revolutionized the diagnosis and investigation of liver hydatid disease. US examination of the liver is readily available and easy to master; it is comparatively cheap and noninvasive, enables interventional procedures, and does not expose the patient to radiation. In areas with endemic hydatidosis, it is the most important single diagnostic tool. On US, HCs are well-defined, circumscribed, cystic lesions with a clear membrane and do not infiltrate surrounding liver tissue. Cysts on examination can be solitary or multiple and univesicular or multivesicular. Pathognomonic US diagnostic features are (a) unmistakable daughter cysts (“rosettes”) within the main cyst cavity, (b) detachment of the membrane of the cyst (“double-contoured membrane”), (c) agglomeration of daughter cysts in the dependent portion of a HC, and (d) calcification of the cyst wall. Because the US pattern reflects the natural history of the HC (evolution vs. involution), accurate interpretation, standardization, and classification of the ultrasound finding can be used for several purposes: (a) screening in endemic areas and family members in infested families, (b) staging and treatment planning, (c) first-line diagnostics, (d) interventional, nonoperative treatment procedures, (e) intraoperative US (IOUS) during surgery, (f) treatment monitoring and postoperative follow-up, and (g) in the workup of patients presenting with jaundice, as it is possible to differentiate daughter cysts from gallstones in the common bile duct (CBD) in most of these patients.
Table 3 The WHO-IWGE Classification of HC | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
US is superior to computerized tomography (CT) in the investigation of the cyst wall, hydatid sand, daughter cysts, and the relationship of the cyst to the diaphragm. Without a clear knowledge and understanding of the disease, accurate interpretation of US findings can be difficult or even impossible. Only “simple HCs” have a uniform, easy-to-interpret US appearance. HCs that have undergone one or more morphologic changes can be a diagnostic problem because of nonspecific US patterns.
Based on US signs, Hassen Gharbi in 1981 classified liver HCs into five types. This classification is still widely used. The cyst types are (a) I, pure fluid collection, (b) II, fluid collection with a split wall, (c) III, fluid collection with septa, (d) IV, heterogeneous appearance, and (e) V, reflecting thick walls. Gharbi cyst types II and III as well as type V calcified cysts are characteristic for liver cystic hydatid disease. In endemic areas, cyst types I and V suggest cystic liver hydatid disease, and cyst type IV can be a diagnostic problem. Different cyst types can coexist in the same patient, reflecting different developmental stages.
The need to more accurately evaluate the functional state of the parasite, especially in field studies, has resulted in a new, modified ultrasound classification of liver HCs. Based on cyst characteristics described in the Gharbi classification, the WHO-IWGE proposed a new classification reflecting the functional state of the parasite that facilitates selection of treatment modalities, as shown in Table 3 and Figure 8.
Based on the WHO classification, certain management guidelines have been suggested. In patients with active cysts (type CL, CE1, CE2), about one-third of the cysts will be sterile. In these patients there are three management options: (a) wait and see, (b) further differential diagnosis, and (c) chemotherapy. The remaining two-thirds of the patients will have fertile cysts and half of them will have secondary cysts. In this group, management modalities are surgery, PAIR, and chemotherapy. Active cysts are the most frequent presentation of liver HCs in the clinical setting. In patients with degenerating (transitional state) cysts (type CE3), living protoscoleces can exist and all treatment options should be considered. In patients with inactive cysts (type CE4 and CE5), further differential diagnosis is frequently necessary. As a rule there are no living protoscoleces in these cysts and, if no complications related to the cyst are present, no treatment is necessary.
Intraoperative Us
In my experience, IOUS can affect the operative strategy. IOUS can be used (a) for “mapping” of the HC (determining its exact position, shape, and its relationship to neighboring structures, e.g., as partially calcified cysts close to hepatic veins and VCI; central cysts close to the hilum), (b) when managing patients with multiple cysts, (c) when the atrophy/hypertrophy complex distorts the anatomy, (d) in reoperations for hydatid disease, (e) in the localization and management of small, nonpalpable cysts deep in liver parenchyma, (f) in the examination of the extrahepatic biliary tree, (g) in detection of cystobiliary communication, which, however, is not always accurate even in experienced hands, and (h) in the search for exogenous vesiculation, especially if nonresectional surgery is performed. An important special technique is that, after partial pericystectomy, the probe should be introduced into the sterilized cyst cavity and the entire inner wall scanned for exogenous vesiculation. An exogenous cyst can be approached and managed directly through the pericyst with minimal risk.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree