Ductal Hyperplasia and Intraductal Carcinoma
DUCTAL HYPERPLASIA—USUAL AND ATYPICAL
The distinction between intraductal hyperplasia and intraductal carcinoma is important for patient management (1). In most instances, intraductal proliferations are readily classified by pathologists, on the basis of generally accepted histopathologic features, as either hyperplasia or in situ carcinoma (2). There exists a small subset for which assignment to either of these categories is less certain. The existence of these “borderline” lesions, which may be diagnosed as atypical hyperplasia or in situ carcinoma, depending upon which criteria are used, is not a compelling reason for abandoning the existing practice of distinguishing pathologically and clinically between ductal hyperplasia and in situ duct carcinoma. Studies of interobserver differences in the diagnosis of highly selected examples of these lesions have focused attention on this troublesome diagnostic problem that applies to a small percentage of proliferative breast changes (3,4,5).
No clinical features are specifically associated with ductal hyperplasia. The alterations caused by epithelial proliferation in individual ducts or in groups of ducts are microscopic in dimension and, consequently, often not palpable. Ductal hyperplasia in various forms is a frequent constituent of “fibrocystic changes” that may be detected by mammography or as a palpable tumor. The lesion complex can also include sclerosing adenosis, cystic and papillary apocrine metaplasia, duct stasis, fibrosis or pseudoangiomatous stromal hyperplasia, and lobular hyperplasia. An important corollary to the lack of clinical indicators of ductal hyperplasia is an inability to determine the duration of these lesions. The date of a ductal hyperplasia biopsy is customarily used as if it were the date of “onset.” This practice, which is a consequence of inability to determine the preclinical duration of hyperplastic ductal lesions, could be a source of bias in assessing the precancerous significance of proliferative lesions in individual patients.
The mammographic manifestations of duct hyperplasia include altered duct patterns, parenchymal distortion, nonpalpable mass lesions, calcification, and asymmetry when both breasts are compared. Calcifications are the most frequent mammographic indication of atypical ductal hyperplasia in the absence of a palpable abnormality (6,7,8). Lesions described on mammography as radial scars often have a component of ductal hyperplasia. Some, but not all, radial scar lesions contain microcalcifications. In the era that preceded the widespread use of mammography, when the indication for biopsy was a palpable abnormality, ductal hyperplasia was found in 25% or fewer of specimens obtained (9,10). No more than 5% of these biopsies had atypical ductal hyperplasia. The frequency of these atypical abnormalities is higher among mammographically directed biopsies, including surgical excisions and stereotactic needle core biopsies (11,12).
Ductal hyperplasia can be found in female patients at virtually any age. After age 60, ductal hyperplasia becomes less frequent, and, when present, the growth pattern is usually less florid than in younger women. However, an occasional woman older than age 60 may be found to have extensive proliferative changes with florid ductal hyperplasia.
Ductal hyperplasia describes a proliferative condition that is manifested histologically as an increase in the cellularity of epithelium in ducts. Because the normal resting epithelium consists of a continuous monolayer of cuboidal-to-columnar epithelial cells supported by a
discontinuous layer of myoepithelial cells, an increase in the cellularity of this two-layer configuration constitutes hyperplasia. The increased thickness of the epithelial layer results in partial or complete obstruction of the duct lumen at the site of the proliferative abnormality. If intraductal hyperplasia is traced in serial sections, it is often possible to observe the discontinuous and multifocal nature of the condition. Various distortions of the basic ductal architecture occur when hyperplastic ducts become more sinuous or are incorporated into complex proliferative lesions, such as papillomas or “radial scars.”
discontinuous layer of myoepithelial cells, an increase in the cellularity of this two-layer configuration constitutes hyperplasia. The increased thickness of the epithelial layer results in partial or complete obstruction of the duct lumen at the site of the proliferative abnormality. If intraductal hyperplasia is traced in serial sections, it is often possible to observe the discontinuous and multifocal nature of the condition. Various distortions of the basic ductal architecture occur when hyperplastic ducts become more sinuous or are incorporated into complex proliferative lesions, such as papillomas or “radial scars.”
The histologic criteria for the diagnosis of duct hyperplasia are the same for needle core biopsy samples and surgical excision specimens. However, needle core biopsy sections present disconnected portions of the lesional area and therefore lack the helpful contextual information provided by intact samples from a surgical biopsy. This situation can lead to overinterpretation of individual ducts seen in isolation.
Pathology of Usual Ductal Hyperplasia
When individual cell borders are inconspicuous, the cellular proliferation in ductal hyperplasia often has a syncytial appearance. Cytoplasmic vacuolization may occur. True intracytoplasmic microlumens that contain secretion that stains positively with the mucicarmine or alcian blue-PAS (periodic-acid-Schiff) stains are extremely uncommon in hyperplastic ductal epithelium (13). The presence of intracytoplasmic mucin-containing microlumens is an atypical feature that should result in careful consideration of a diagnosis of intraductal carcinoma or pagetoid lobular carcinoma in situ. In duct hyperplasia nuclear spacing is uneven so that in some areas the cells appear crowded and the nuclei seem to overlap. Nucleoli are inapparent or inconspicuous unless there is a prominent element of apocrine metaplasia. Mitotic figures are very infrequent and when present they have a regular configuration.
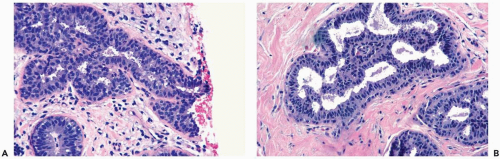
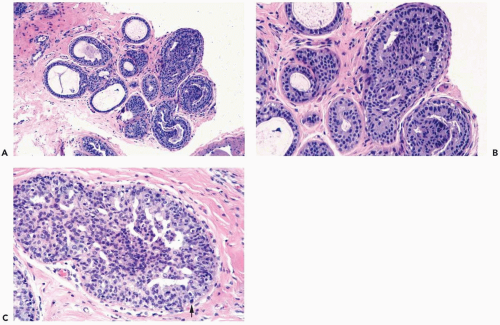
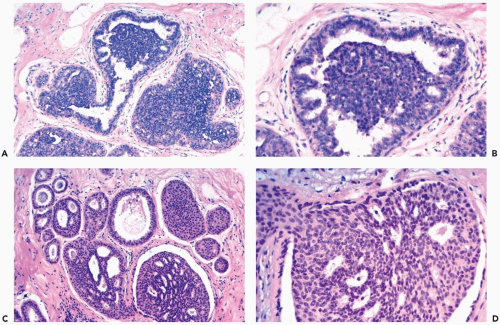
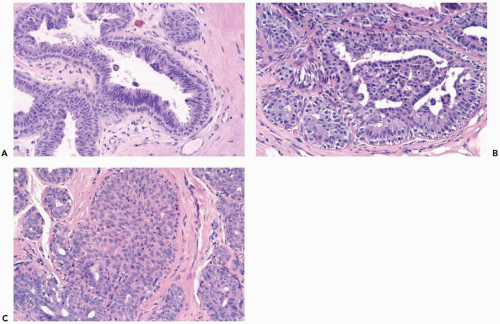
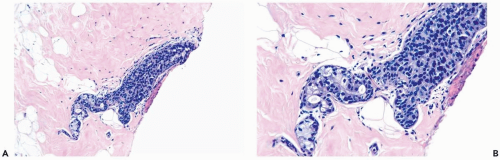
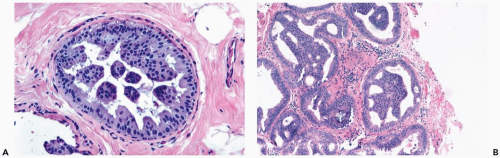
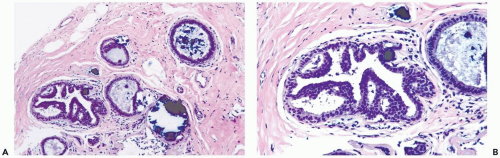
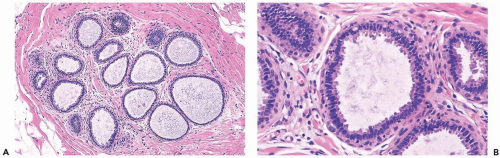
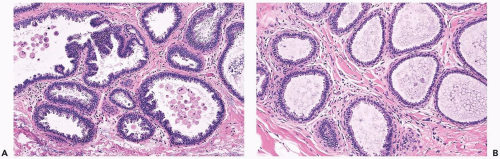
Mild hyperplasia may affect the entire epithelium circumferentially in a duct cross section or only a segment of the duct. It occurs as an increase in the amount of epithelium, which rarely exceeds three cells in thickness. The epithelium may be focally papillary (Fig. 8.1).
In moderate hyperplasia, the epithelium tends to be more than three cells in thickness. Intraluminal proliferation is more pronounced than in mild hyperplasia, resulting in the formation of secondary glandular lumina (Fig. 8.2). Part of the duct lumen may remain as a crescentic space or spaces at the edge of the duct in cribriform hyperplasia (Fig. 8.3). Micropapillary hyperplasia is part of the spectrum of mild and moderate ductal hyperplasia (Fig. 8.4). The papillae are blunt or slender, irregularly shaped fronds of epithelium in which the apical cells are smaller and have more condensed nuclei than those in the adjacent basal epithelium. See Chapter 10 for a discussion of columnar cell duct hyperplasia and tubular carcinoma.
The nuclei in moderate hyperplasia are often overlapping, irregularly shaped, and may be distributed in a “streaming” fashion (Fig. 8.5). Streaming refers to a growth pattern in which the nuclei of hyperplastic epithelial cells are oriented parallel to the long axes of the cells (Fig. 8.6). Because the cytoplasmic borders of these cells are often indistinct, streaming is usually detected as a parallel orientation of oval or spindle-shaped nuclei. Streaming occurs in most structural patterns of usual and atypical intraductal hyperplasia. The association of the streaming pattern with ductal hyperplasia has been confirmed by computerized morphometric analysis of the orientation of nuclei in proliferative duct lesions (14).
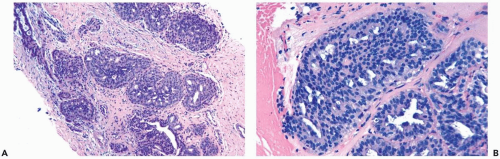
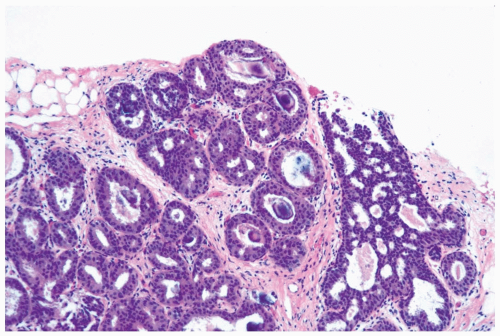
The distinction between moderate and florid hyperplasia is not sharp, but lesions are generally placed in the latter category when the affected ducts are appreciably enlarged in comparison with nonhyperplastic counterparts. Florid hyperplasia has the papillary and bridging growth patterns that are encountered in moderate hyperplasia, but the
overall proliferation tends to be more cellular and complex than in moderate hyperplasia (Fig. 8.7). Foci of florid hyperplasia are more likely to fill the entire duct lumen in a solid or fenestrated (cribriform) fashion.
overall proliferation tends to be more cellular and complex than in moderate hyperplasia (Fig. 8.7). Foci of florid hyperplasia are more likely to fill the entire duct lumen in a solid or fenestrated (cribriform) fashion.
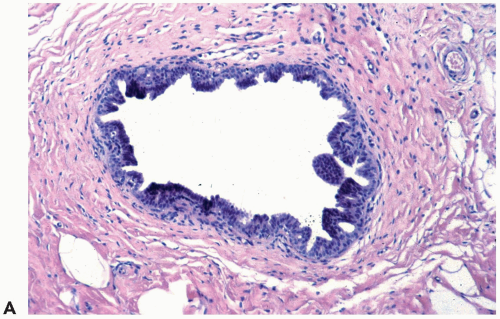 Figure 8.4 Ductal hyperplasia, micropapillary. A. The micropapillary epithelium consists of columnar cells with dark, condensed nuclei and scant cytoplasm. |
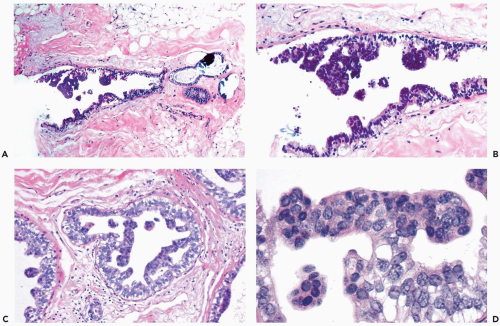
Necrotic cellular debris is rarely present in hyperplastic ducts, and when found it is usually associated with florid sclerosing papillary hyperplasia (Fig. 8.8). In such lesions, the hyperplastic ducts with necrosis are indistinguishable cytologically and structurally from adjacent ducts with nonnecrotic hyperplastic epithelium. Histiocytes or foam cells are found relatively often in the lumens of hyperplastic ducts, and the presence of these cells should not be mistaken for necrosis (Fig. 8.9).
The fenestrated growth pattern that occurs in moderate and severe ductal hyperplasia results from the formation of epithelial bridges that become joined as they traverse the
duct lumen. The fenestrations represent portions of the original duct lumen that has been passively subdivided by the complex arborizing epithelial proliferation. Using a serial section, three-dimensional reconstruction method, Ohuchi et al. (15) demonstrated that the lumina that appear to be separated from each other in a two-dimensional histologic section of papillary intraductal hyperplasia are actually part of a network of channels surrounded by the proliferating epithelium. By contrast, three-dimensional reconstruction of intraductal carcinoma revealed that the fenestrations in these lesions are newly formed, disconnected spaces bordered by polarized neoplastic cells.
duct lumen. The fenestrations represent portions of the original duct lumen that has been passively subdivided by the complex arborizing epithelial proliferation. Using a serial section, three-dimensional reconstruction method, Ohuchi et al. (15) demonstrated that the lumina that appear to be separated from each other in a two-dimensional histologic section of papillary intraductal hyperplasia are actually part of a network of channels surrounded by the proliferating epithelium. By contrast, three-dimensional reconstruction of intraductal carcinoma revealed that the fenestrations in these lesions are newly formed, disconnected spaces bordered by polarized neoplastic cells.
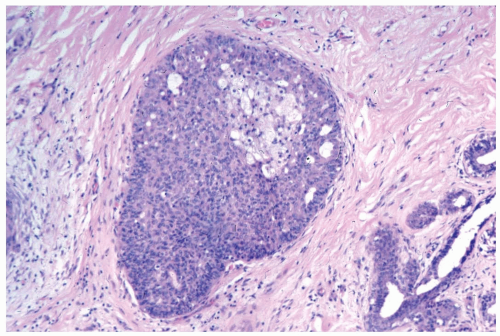 Figure 8.9 Ductal hyperplasia, florid with histiocytes. The epithelial proliferation in this enlarged duct is solid with peripheral fenestrations. Histiocytes are present in the duct. |
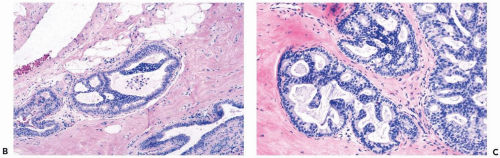
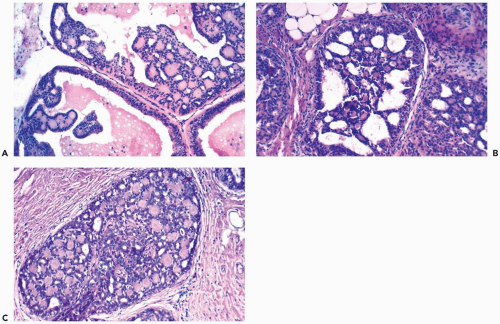
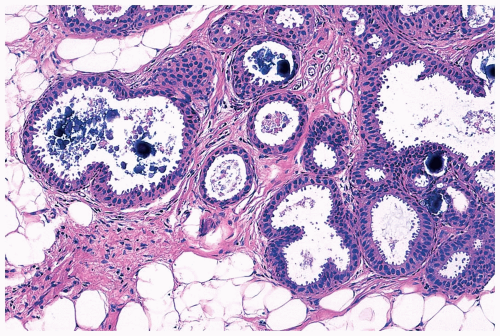
The spaces that are found in histologic sections of fenestrated intraductal hyperplasia have distinctive features. The secondary lumens tend to be larger and more numerous at the periphery of the duct than centrally, but the reverse distribution may be encountered. Cells outlining these spaces are distributed in a haphazard fashion, except at the edge of the duct, where residual columnar or cuboidal duct epithelium composed of cells with more regularly oriented nuclei may persist. The spaces in a given duct usually have varied shapes (ovoid, crescentic, irregular, or serpiginous) rather than being rounded as they tend to be in cribriform carcinoma (Figs. 8.3, 8.5, 8.6, 8.7, 8.10). The spaces formed in intraductal hyperplasia may be empty or they may contain secretion and histiocytes. Fine calcifications can develop in the glandular lumina of intraductal hyperplasia.
A layer of myoepithelial cells is often evident at the edges of hyperplastic ducts in H & E sections (Fig. 8.11). These cells may accompany the proliferation into the duct lumen when the fibrovascular stromal framework of papillary hyperplasia is present. Immunohistochemical stains are extremely useful for highlighting the myoepithelium in proliferative ductal lesions. Experience has led to the conclusion that the reactivity of individual markers is unpredictable in a given case and that it is advantageous to use at least three myoepithelial markers.
p63 is the only marker currently available that is localized specifically in the nuclei of myoepithelial cells. It is not reactive with myofibroblasts or blood vessels (16). p63 is rarely reactive with scattered epithelial cell nuclei in papillary lesions and duct hyperplasia. These epithelial cells can be distinguished from myoepithelial cells by their cytologic appearance or their position. Nuclear staining for p63 produces a string of dots around the epithelium in benign ducts and lobules.
Several cytoplasmic markers are available for highlighting the myoepithelium: smooth muscle actin (SMA), smooth muscle myosin heavy chain (SM-MHC) calponin, CK5 and CD 10. These markers exhibit variable cross reactivity with myofibroblasts or blood vessels (16,17,18,19). Myofibroblastic reactivity is greatest with SMA or calponin and least with CD10, also referred to as the common acute lymphoblastic leukemia antigen or CALLA. However, many exceptions can be encountered. For this reason, as well as the variable reactivity of these reagents with myoepithelial cells, it is prudent to use two or more cytoplasmic markers as well as p63 to evaluate the myoepithelium in a given case.
Myoepithelium is highlighted with immunostains in normal ducts and lobules. When proliferative fibrocystic changes occur in these structures, such as adenosis or duct hyperplasia, the myoepithelium is usually uniformly present. Attenuation of myoepithelial cells that occurs in some hyperplasias, especially sclerosing papillary lesions and duct hyperplasia with atypia, results in increased space between p63 reactive nuclei when compared to the staining pattern in normal structures. In this situation, the integrity of the myoepithelium can usually be demonstrated with one of the cytoplasmic markers for these cells. Because of the stromal proliferation that accompanies many of these lesions, care must be taken not to mistake myofibroblastic reactivity for myoepithelium. Because myoepithelium persists in some examples of intraductal carcinoma, where it can be attenuated or hyperplastic, the presence of this cell layer is not specific for distinguishing between hyperplasia and intraductal carcinoma. On the other hand, an intraductal
proliferation devoid of myoepithelium that is confirmed with more than one marker, in the presence of internal positive controls, is almost certainly in situ carcinoma.
proliferation devoid of myoepithelium that is confirmed with more than one marker, in the presence of internal positive controls, is almost certainly in situ carcinoma.
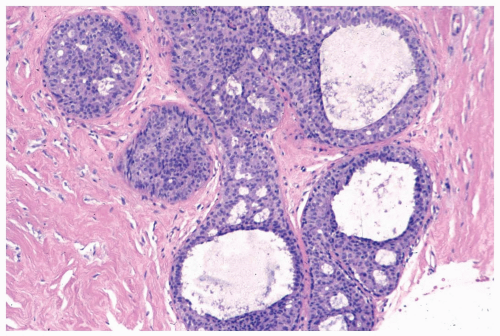 Figure 8.10 Ductal hyperplasia, florid. Microlumina and the main duct lumina are apparent in these ducts in a needle core biopsy specimen. |
Collagenous spherulosis is a special form of duct hyperplasia wherein myoepithelial cells contribute to the formation of nodular subepithelial deposits of basement membrane material (Fig. 8.12).
Pathology of Atypical Ductal Hyperplasia
A broad agreement stands on the general description of atypical ductal hyperplasia as a proliferative lesion that fulfills some but not all criteria for a diagnosis of intraductal carcinoma. The difficulty in arriving at a more crisp definition lies in the specifics. In general, these can be considered under two headings: quantitative and qualitative. The former refers to the amount of a proliferative abnormality, whereas the latter is concerned with microscopic structural and cytologic details.
Quantitative criteria for distinguishing between ductal hyperplasia and intraductal carcinoma have been based on the number of duct cross sections that exhibit the abnormality or the dimension of the affected area. Some investigators have classified proliferative lesions limited to a single duct as atypical ductal hyperplasia, even if the abnormality is qualitatively consistent with intraductal carcinoma (10). Based on the criterion requiring at least two fully involved duct cross sections for a diagnosis of intraductal carcinoma, cases are arbitrarily assigned to the category of atypical hyperplasia when only one qualitatively diagnostic duct is present.
Another scheme emphasizes the microscopic dimensions of a lesion as one of the bases for a diagnosis of atypical ductal hyperplasia (20). According to these criteria, foci measuring less than 2 mm are diagnosed as atypical ductal hyperplasia, regardless of the number of duct cross sections, even if the individual ducts qualify as intraductal carcinoma. The 2-mm criterion was selected because “it was at the level of one or more small ducts or ductules measuring around 2 mm in aggregate cross-sectional diameter that most pathologists felt hesitant in diagnosing a lesion as intraductal carcinoma” (21). Another explanation offered by the proponents of this criterion was that “questions about quantity are raised generally when dispersed lesions add up to from 1.6 to 2.7 mm in aggregate size. Therefore, we arbitrarily chose 2 mm as a cutoff point” (20).
No scientific studies have compared the clinical significance of different quantitative criteria for the diagnosis of atypical duct hyperplasia. No a priori reason exists for choosing two duct cross sections or 2 mm as critical decision points in relation to risk. For example, no data exist for the risk to develop subsequent carcinoma in patients whose biopsies contained proliferative lesions qualitatively consistent with intraductal carcinoma limited to one-,
two-, or three-duct cross sections, respectively. Regarding the dimensions of these lesions, no analysis comparing foci measuring 1.5 mm, 2.0 mm, 2.5 mm, or larger has been reported.
two-, or three-duct cross sections, respectively. Regarding the dimensions of these lesions, no analysis comparing foci measuring 1.5 mm, 2.0 mm, 2.5 mm, or larger has been reported.
Several technical issues hamper the application of quantitative criteria, especially in the diagnosis of needle core biopsy findings. What appear to be two contiguous cross sections may prove in serial sections to be part of a single duct, or deeper sections of what appears to be a single duct lesion may uncover additional involved duct cross sections. How close must two duct cross sections be to be considered contiguous? Is stroma between duct cross sections included in the measurement? Quantitative criteria assume that the ducts in question have been sectioned transversely. How to assess ducts cut longitudinally has not been adequately addressed. If the longitudinal dimension of a duct in a section exceeds 2 mm but the transverse diameter is 1 mm, should this focus be considered intraductal carcinoma when using the 2-mm criterion?
Others have rejected quantitative factors in the diagnosis of atypical ductal hyperplasia. This position was elaborated by Fisher et al. (22), who stated that “our definition of atypical ductal hyperplasia consists of a ductal epithelial alteration approximating, but not unequivocally satisfying, the criteria for a diagnosis of ductal carcinoma in situ (DCIS). It does not include arbitrarily established quantities of unequivocal DCIS (less than 2.0 mm or 2 ‘spaces’).” In their study of the prognostic significance of proliferative breast “disease,” Bodian et al. (9) reported that “during the course of many years, intraductal carcinoma has been diagnosed if the characteristic features are present in only one ductal space.”
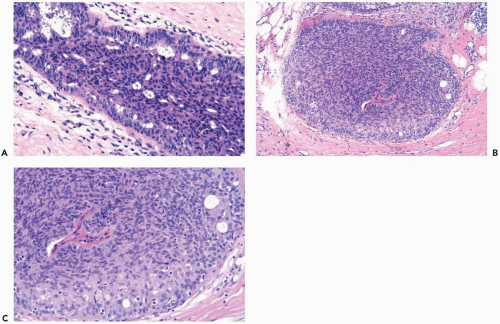
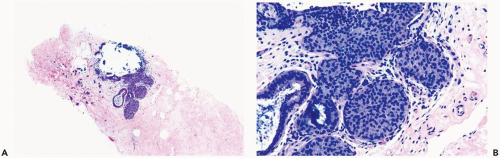
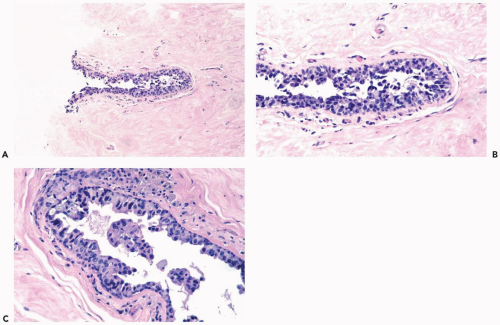
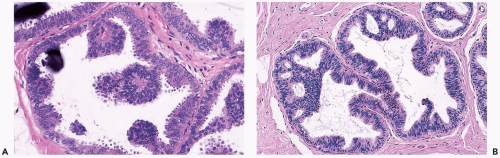
The role of quantitative factors in the diagnosis of proliferative ductal lesions seems to lie between these extremes. The use of rigid criteria such as two-duct cross sections or 2 mm, can be justified in a research setting to ensure a homogeneous study group or to assess a particular criterion, but the strict application of these arbitrary rules in a clinical setting is difficult for technical reasons stated above and poorly substantiated by existing data. Given the limitations of current methods for diagnosing intraductal lesions, quantitative factors sometimes play a role in the assessment of a particular lesion in material obtained in a needle core biopsy. This situation arises when the biopsy contains detached fragments of cytologically atypical epithelium (Fig. 8.13), or when only part of one duct with changes suggestive of intraductal carcinoma is represented (Figs. 8.14, 8.15, 8.16, 8.17). The same issue arises when a process that suggests lobular extension of ductal carcinoma is present (Fig. 8.18).
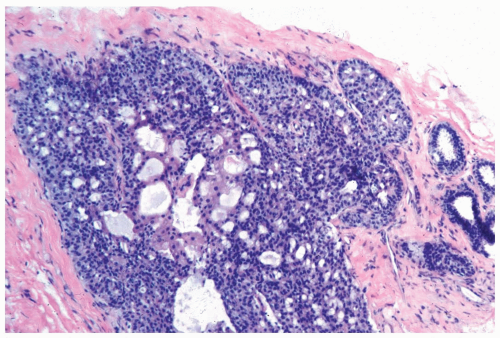
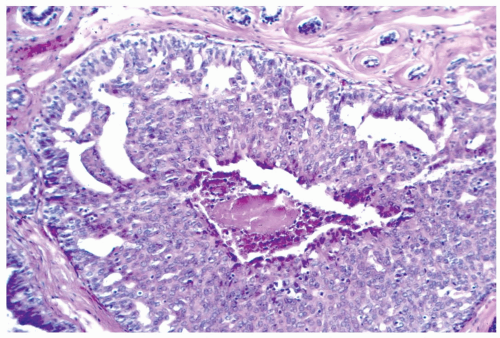
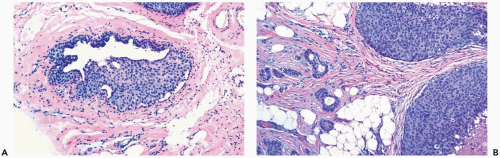
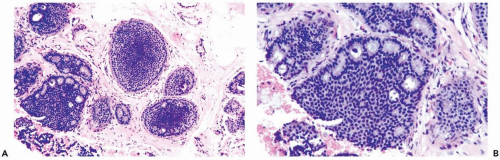
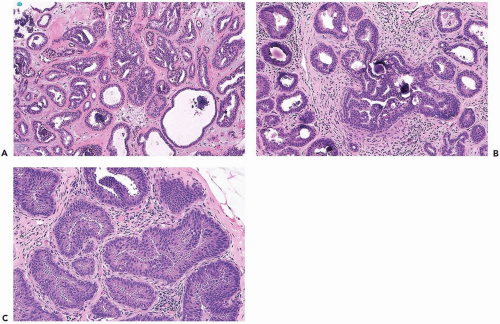
In many instances, the diagnosis of atypical duct hyperplasia depends upon the presence of structural elements of intraductal carcinoma mingling with hyperplasia. Architecturally, this may be manifested by a cribriform pattern
partially involving a duct (Figs. 8.19, 8.20). Atypical hyperplasia can be encountered in ducts exhibiting apocrine metaplasia (Figs. 8.21, 8.22). Cytologic atypia may involve individual cells, groups of cells, or the entire population of a proliferative lesion. Atypical cytological features include nuclear enlargement with an increased nuclear to cytoplasmic ratio, nuclear hyperchromasia, an irregular chromatin pattern, or the presence of enlarged, pleomorphic nucleoli (Fig. 8.23).
partially involving a duct (Figs. 8.19, 8.20). Atypical hyperplasia can be encountered in ducts exhibiting apocrine metaplasia (Figs. 8.21, 8.22). Cytologic atypia may involve individual cells, groups of cells, or the entire population of a proliferative lesion. Atypical cytological features include nuclear enlargement with an increased nuclear to cytoplasmic ratio, nuclear hyperchromasia, an irregular chromatin pattern, or the presence of enlarged, pleomorphic nucleoli (Fig. 8.23).
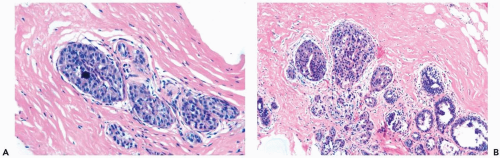
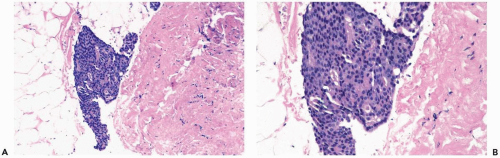 Figure 8.13 Atypical ductal hyperplasia. A, B. This needle core biopsy specimen included a detached fragment of nearly solid proliferative duct epithelium. |
The most challenging atypical ductal proliferations, sometimes referred to as “borderline” lesions, feature marked cytologic and architectural atypia. Most of these foci retain a minor characteristic of hyperplasia, such as persistent columnar ductal epithelium or nuclear overlap, with a structure otherwise typical of intraductal carcinoma (Figs. 8.23, 8.24, 8.25). These slight variations will be disregarded by observers who classify such lesions as intraductal carcinoma, whereas others may diagnose atypical hyperplasia. Similarly, those who place credence in quantitative criteria will diagnose atypical hyperplasia because the extent of a lesion is not sufficient, whereas others, not adhering to these rules, will diagnose intraductal carcinoma.
Insufficient emphasis has been placed on diagnosing specific proliferative lesions in the context of the overall spectrum of histologic changes in a biopsy specimen. In a research setting, a pathologist can be required to make a diagnosis that is based only on one or more selected foci on a single slide. This situation, duplicated to a large extent in assessing needle core biopsies, is different from the circumstances under which the various diagnostic criteria were originally developed, in which multiple histologic sections were reviewed (10,20,22). When faced with an atypical ductal proliferative lesion in a needle core biopsy specimen, the
pathologist should obtain serial sections of the specimen and, if they are available, slides from previous biopsies should be reviewed for comparison. The diagnosis of “borderline” intraductal proliferations is best made in the context of the spectrum of pathologic changes present in current and prior specimens. A focus of concern may be found to be substantially more atypical and different qualitatively from the overall proliferative level in a given case, or it may prove to be part of a spectrum of changes lacking distinct histologic boundaries. The former situation would tend to support a diagnosis of intraductal carcinoma in the lesional area, whereas the latter suggests atypical hyperplasia.
pathologist should obtain serial sections of the specimen and, if they are available, slides from previous biopsies should be reviewed for comparison. The diagnosis of “borderline” intraductal proliferations is best made in the context of the spectrum of pathologic changes present in current and prior specimens. A focus of concern may be found to be substantially more atypical and different qualitatively from the overall proliferative level in a given case, or it may prove to be part of a spectrum of changes lacking distinct histologic boundaries. The former situation would tend to support a diagnosis of intraductal carcinoma in the lesional area, whereas the latter suggests atypical hyperplasia.
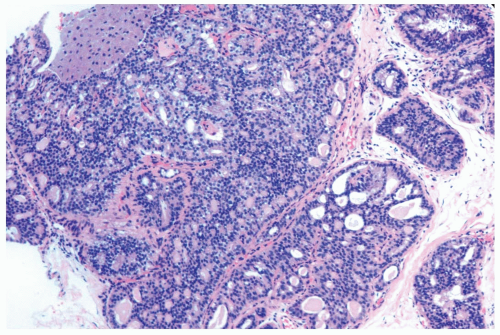 Figure 8.20 Atypical ductal hyperplasia, cribriform. The florid atypical hyperplasia in this needle core biopsy specimen from a solid papillary lesion has areas of cribriform microlumen formation. |
Columnar Cell Lesions
Proliferative alterations of the duct-lobular complex have become the subject of closer scrutiny as a result of the widespread use of needle core biopsy to sample mammographically
detected lesions. Lubelsky et al. (23) reported that 21% of needle core biopsy specimens obtained for calcifications in mammographically screened women had columnar cell lesions. These abnormalities are now recognized as being part of a spectrum of lesions described by the terms columnar cell change and columnar cell hyperplasia (CCH) (24,25). Other more cumbersome names that have been offered include atypical cystic lobules (26), cancerization of lobules and atypical ductal hyperplasia adjacent to ductal carcinoma in situ (27) and columnar alteration with prominent apical snouts and secretions, or CAPSS (28).
detected lesions. Lubelsky et al. (23) reported that 21% of needle core biopsy specimens obtained for calcifications in mammographically screened women had columnar cell lesions. These abnormalities are now recognized as being part of a spectrum of lesions described by the terms columnar cell change and columnar cell hyperplasia (CCH) (24,25). Other more cumbersome names that have been offered include atypical cystic lobules (26), cancerization of lobules and atypical ductal hyperplasia adjacent to ductal carcinoma in situ (27) and columnar alteration with prominent apical snouts and secretions, or CAPSS (28).
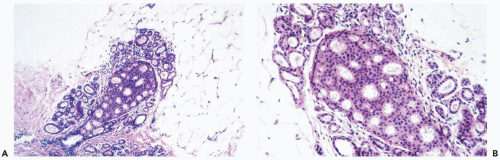
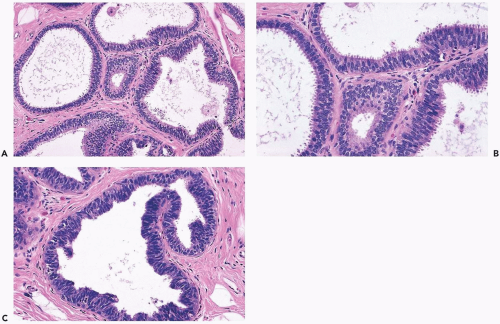
Columnar cell hyperplasia is a multifocal process that may also be bilateral. It is most often encountered in women ages 35 to 50, but CCH can be present after the menopause. CCH rarely produces a palpable abnormality, and it is usually detected mammographically because calcifications are frequently formed, becoming the target of needle core biopsy sampling. The fundamental lesion is localized in terminal duct-lobular units that become enlarged as a result of cystic dilatation.
The simplest form of this process features a thin, flat epithelial layer composed of cuboidal to tall columnar cells distributed in a relatively uniform pattern. The cells appear crowded and dark. The apical cell surface usually has an apocrine-type cytoplasmic protrusion (“snout”) and, in some cases, this is a prominent feature. In the most banal columnar cell lesion, which is best termed columnar cell change, the epithelium is one to two cells deep and there is little nuclear pleomorphism. Nucleoli and mitotic figures are very rarely found or absent (Fig. 8.26). When present, calcifications appear in the form of amorphous granular material or discrete basophilic deposits (Fig. 8.27).
The cytologic features of columnar cell lesions suggest apocrine differentiation. The cells express gross cystic disease fluid protein-15, an apocrine marker. Ki67 immunoreactivity is low or absent, even in hyperplastic foci (29). The epithelium is also immunoreactive for bcl-2 and estrogen receptor protein, which are typically absent in benign apocrine change (29).
Columnar cell hyperplasia is present when the epithelium is more than two-cells thick. This is most readily apparent when cellular crowding becomes pronounced and nuclei are not distributed in a single plane relative to the basement membrane. This tendency to “stacking” of nuclei is usually accompanied by increased nuclear chromasia, and small mounds may be formed in the most cellular regions (Fig. 8.28).
More complex columnar cell proliferative foci compromise lesions described as columnar cell hyperplasia with atypia. Mild atypia is usually manifested by the presence of small and often isolated foci of micropapillary growth in a background of otherwise usual CCH (Fig. 8.29). The presence of more elaborate growth patterns as well as cytologic atypia characterize columnar cell hyperplasia with moderate to marked atypia, which in its most severe form approaches the appearance of intraductal carcinoma (Figs. 8.30, 8.31). In some instances, cytologic atypia is more pronounced than the structural abnormalities (Fig. 8.32). When carcinoma arises in columnar cell hyperplasia, the growth pattern is usually one of the characteristic forms of intraductal carcinoma (Figs. 8.33, 8.34), but rarely so-called “flat micropapillary” intraductal carcinoma with relatively little structural complexity is encountered (Fig. 8.35). Atypical lobular hyperplasia and lobular carcinoma in situ frequently accompany columnar cell abnormalities, and tubular carcinoma may also be
present (Fig. 8.36). Hence, columnar cell lesions are part of a triad that includes lobular neoplasia and tubular carcinoma. All components of this triad are not present in every case.
present (Fig. 8.36). Hence, columnar cell lesions are part of a triad that includes lobular neoplasia and tubular carcinoma. All components of this triad are not present in every case.
Columnar cell lesions develop calcifications that are formed in multiple glands in many of the proliferative sites. Two types of calcifications are encountered: crystalline and ossifying. The crystalline type, usually associated with lesions with less atypia, is deeply basophilic, opaque, round or angular, and prone to fragmentation in the process of histologic sectioning (Figs. 8.27, 8.30, 8.31, 8.33). An ossifying type of calcification usually has a rounded, well-defined contour, and an internal structure that resembles an ossifying nodule in which basophilic granular calcific deposits are embedded in lacunar-like spaces with an orangophilic or eosinophilic matrix (Figs. 8.33, 8.37). Ossifying type calcifications occur throughout the range of columnar cell hyperplasias, and they appear to develop in the proliferative epithelium, whereas basophilic crystalline deposits are predominantly intraluminal. Both types of calcification can be found in one specimen, and they may occur together in a single proliferative focus.
Excisional biopsy is recommended when a needle core biopsy contains columnar cell hyperplasia with atypia, or if a columnar cell lesion is associated with lobular carcinoma in situ or atypical lobular hyperplasia. Guerra-Wallace et al. (30) evaluated patients who underwent surgical excision after a columnar cell lesion was found in a needle core biopsy specimen. They reported finding carcinoma in 10 of 135 (7.4%) of women with columnar cell hyperplasia without atypia and in 11 of 60 (18.3%) with coexisting atypical hyperplasia. No studies exist that specifically have investigated the long-term follow-up of women with columnar cell hyperplasia with atypia (31).