Ductal Carcinoma In Situ
SYED A. HODA
HISTORICAL BACKGROUND
Ductal carcinoma in situ (DCIS), a term that is synonymous with intraductal carcinoma, was defined pathologically early in the 20th century largely by surgeons interested in the microscopic study of tumors they encountered clinically. In situ, Latin for “in its place,” was first used as a term for noninvasive malignancy by Broders in 1932.1 Among the first studies of DCIS were those of Warren,2 a surgeon practicing in Boston. Warren’s2 investigation of “abnormal involution” or cystic disease led him to conclude that carcinoma might develop by transition from hyperplastic duct lesions:
“It is precisely under these conditions that we most frequently find the combination of abnormal involution and carcinoma. The transition stage is observed when the epithelium no longer confines itself to the cyst cavity, but breaks through the limiting membrane and infiltrates the adjacent structures.”
Fundamental pathologic and clinical studies of proliferative ductal lesions of the breast were performed during the early decades of the 20th century by two other surgeons, Sir G. Lenthal Cheatle of King’s College Hospital, London, and Joseph Colt Bloodgood (a disciple of Halsted, of the eponymous mastectomy fame) of Johns Hopkins University Hospital, Baltimore, Maryland. The extent to which Bloodgood and Cheatle influenced each other is difficult to ascertain from their published articles that rarely contained references to work other than their own. Contemporaries separated by the Atlantic Ocean, which was figuratively much wider at the time, they seem to have pursued independent routes in their efforts to more clearly distinguish between benign and malignant lesions of the breast.
Cheatle drew heavily upon his own detailed studies of whole-organ sections of the breast to examine the relationships of various lesions to carcinoma as part of a systematic exploration of pathologic processes in the breast. Bloodgood’s approach was case oriented, dealing largely with an analysis of patients under his personal care at Johns Hopkins University over several decades. Because of his concern with diagnostic and therapeutic problems prevailing in the operating room at the time, much of Bloodgood’s attention was directed to biopsy specimens. He was, therefore, able to relate the morphology of many lesions to clinical follow-up, sometimes of the unresected breast.
Early descriptions of DCIS outlined the major structural patterns of the disease that are recognized today. Micropapillary DCIS was illustrated by Cheatle3 in 1920 and by Bloodgood4 in 1921, but this term was not used by either author. Bloodgood5 also drew attention to the problem of distinguishing between “borderline” hyperplastic lesions and DCIS. Cheatle referred to the micropapillary proliferation as laciform and noted the “cartwheel” appearance of carcinoma in a nearby duct. Today, many would describe the “cartwheel” focus as cribriform. Muir6 attributed the term cribriform to Schultz-Brauns’7 article on breast carcinoma contained in Henke and Lubarsch’s 1935 Handbook.
The existence of “comedo” (a term that generally refers to high-grade solid type of DCIS with central necrosis) and cribriform patterns of DCIS is readily apparent in Bloodgood’s8 picture, which illustrated a tumor classified as a comedocarcinoma. The accompanying description provides an interesting historical vignette:
In 1893, forty-one years ago, I assisted Dr. Halstead in exploring a clinically benign tumor of the breast. The patient was sixty-seven years of age and had observed a small tumor for about eleven months …. The moment we cut into and pressed on it, there extruded from its surface many grayishwhite, granular cylinders, which I called at that time comedos. From the gross appearance the tumor was diagnosed as malignant, and the radical operation was performed. The nodes were not involved … [and] the patient lived nineteen years after operation, dying at age eighty-six.8
Bloodgood recognized two types of “comedoadenocarcinoma,” which he referred to as “pure comedoadenocarcinoma and comedoadenocarcinoma with areas of fully developed cancer of the breast,” the former being entirely intraductal and the latter partly invasive. He observed that large tumors with gross comedo features were more likely to be in the invasive category. Follow-up revealed that 30% of node-negative patients with invasive comedoadenocarcinoma developed metastases and died of the disease.
Bloodgood’s8 1934 paper described a patient who had a remarkable clinical course. One-year following treatment by excision alone in 1896, the patient developed recurrent carcinoma at the site of prior surgery. A radical mastectomy was then performed. The lymph nodes were negative, and the patient lived more than 15 years without additional recurrence. Because of the apparent curability of the “pure
comedo tumor,” Bloodgood preferred the term comedoadenoma. Treatment by local excision was recommended “when the palpable tumor is small and can be completely excised by cutting through normal breast tissue and closing the wound without injury to the symmetry of the breast.”8
comedo tumor,” Bloodgood preferred the term comedoadenoma. Treatment by local excision was recommended “when the palpable tumor is small and can be completely excised by cutting through normal breast tissue and closing the wound without injury to the symmetry of the breast.”8
This ranks as one the earliest descriptions of breast conservation surgery for DCIS. Needle aspiration cytologic examination was used by Bloodgood for the diagnosis of breast tumors, especially so that “older women may be spared the complete operation for cancer by an aspiration biopsy, when pure comedo tumor involving a large part of, or the entire breast, is recognized.”8 However, he found that aspiration cytology could not be relied upon for making a distinction between intraductal and invasive carcinoma. In the case of a woman with 1.5-cm lesion,
The tumor had been aspirated before it was explored and from examination of the stained aspirated cells we could only decide that they suggested a malignant tumor. We did not recognize the comedo tumor.8
In 1938, Lewis and Geschickter9 described 40 patients treated for comedocarcinoma, reporting an 85% 5-year cure rate, with most 5-year survivors having remained well for 10 years. Included were eight women whose initial treatment was only local excision. Six of these eight women developed recurrent carcinoma within 1 to 4 years. Unfortunately, the authors did not distinguish between lesions that were entirely intraductal and those with concomitant invasive carcinoma.
Until about 30 years ago there was little clinical interest in the histologic subtypes of DCIS. This situation most likely derived from the fact that almost all patients were treated by mastectomy and the observation that the lesions rarely consisted of a single growth pattern, for as Cheatle10 observed, “whole sections reveal that all these varieties may occur in the same mass of disease.”
Those interested in the history of DCIS, a disease that has seen an exponential increase in incidence and prevalence in recent decades, will find Fechner’s11 overview to be particularly helpful.
Concepts regarding various aspects of DCIS continue to evolve. Indeed, some observers find it difficult to accept the intraductal neoplastic proliferation of cells as “carcinoma,” advocate the use of the acronym DIN, that is, ductal intraepithelial neoplasia, and argue that since N and M categories are not typically applicable for these lesions, there is no reason to retain them within the tumor (size), regional node (involvement), (distant) metastases (TNM) system.12 Perhaps, as a reflection of the inherent weakness of this argument, the DIN terminology has not gained wide acceptance (and has been eliminated from the latest WHO13 classification of mammary neoplasms).
CLINICAL PRESENTATION
Frequency
Approximately one-quarter of newly diagnosed breast carcinomas are noninvasive. By American Cancer Society’s estimation, there will be 63,300 new cases of DCIS in the United States in 2012; during the same year, there will be 226,870 new cases of invasive breast carcinoma, and about 39,510 women will die of the disease.14 In 1975, 5.8 American women per 100,000 women were diagnosed with DCIS, and in 2012, the age-adjusted incidence rate of DCIS was 32.5 women per 100,000.15
The reported frequency of DCIS in different studies is influenced by clinical circumstances. A review of approximately 1,000 consecutive women treated at a cancer center in the United States in the late 1970s revealed that 5% had DCIS.16 Data from nine population-based registries included in the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) program for 1975 indicated that 2.9% of patients had DCIS.17 A review of SEER data published in 1996 demonstrated a striking increase in the incidence of DCIS after 1983.18 This change was observed in all age groups. Among women 30 to 39 years of age, the average annual increase in the incidence rate changed from 0.3% between 1973 and 1983 to 12.0% between 1983 and 1992. Similar increases were found for women 40 to 49 years of age (0.4% to 17.4%) and for women 50 years and older (5.2% to 18.1%). The estimated total number of cases of DCIS in 1992 was 200% higher than expected based on 1983 rates. In this series, the anatomical distribution of DCIS in the breast was similar to that of invasive carcinomas, with 44% of lesions in the upper-outer quadrant. Further analysis of the SEER data indicated that the estimated number of new cases of DCIS in 1993 was 23,275.19 Approximately 4,676 were in women 40 to 49 years of age, representing about 15% of breast carcinoma in this age group.
The National Cancer Database reported in 1997 that 3.7% of 31,930 breast carcinomas registered were classified as intraductal.20 The percentage rose to 7.0% and 9.5%, respectively, in 1990 (65,255 cases) and 1993 (93,915 cases). During the same period, the reported frequency of lobular carcinoma in situ (LCIS) was stable, accounting for 1.3% to 1.6% of cases annually.
A population-based study of Danish women in the 1980s revealed that 4% of newly diagnosed carcinomas were intraductal.21 Review of the records of the Connecticut Tumor Registry revealed a yearly increase in the reported number of patients with DCIS.22 In 1979, the 33 diagnoses of DCIS represented 1.8% of breast carcinomas, and in 1988 the 200 cases constituted 7.4% of breast carcinomas. Data from the New Mexico Tumor Registry revealed stable incidence values for DCIS in Hispanic White, non-Hispanic White, and Native American women for more than a decade before 1984.23 Thereafter, the incidence rate increased annually in each ethnic group. In 1994, the incidence rates per 100,000 were 13.8, 9.7, and approximately 7.0, respectively, for non-Hispanic White, Hispanic White, and Native American women. The lower incidence rates in the latter groups may reflect less access to mammography rather than intrinsic ethnic differences in the biology of DCIS. African Americans with DCIS have higher rates of breast carcinoma recurrence, as well as mortality. Analyses of SEER data show that overall mortality is 35% higher in African American versus Caucasian women. The risk of advanced invasive carcinoma was 130% higher in Hispanic and 170% higher in African American versus Caucasian women with DCIS.24
The increased age-adjusted incidence of in situ breast carcinoma in the United States coincides with a leveling off in the overall age-adjusted incidence of invasive carcinoma and of localized carcinoma, and a decline in the incidence of invasive carcinoma classified as “regional.”25 These changes in incidence by stage have been accompanied by a significant decline in age-adjusted breast carcinoma mortality.25 The beneficial effects of mammography as a diagnostic or screening modality, and of improved systemic therapy, are reflected in these trends.
Although the incidence of DCIS has steeply escalated over the last few decades, this rise has not been uniform across various histologic types: low-grade DCIS has accounted for the majority of the recent increase in incidence owing to enhanced radiologic detection, whereas the incidence of highgrade DCIS has remained stable.26
Risk Factors
Data on epidemiologic risk factors specific to DCIS are limited.27,28 There appear to be some age-related differences in associations, but overall the risk factors for DCIS and invasive carcinoma appear to be similar.27 The risk for both lesions increases with age, an association that is stronger for invasive carcinoma. Risk factors for incident DCIS include positive family history.29 BRCA (breast cancer [gene]) mutations were found in 13% of women with DCIS diagnosed before 50 years of age.30 In the largest analysis of DCIS patients in non-Ashkenazi Jewish women, the prevalence of a BRCA1/2 mutation was 5.9%.31 The risk was significantly higher among women younger than 50 years with a personal and family history of breast carcinoma than those 50 years or older.
Mammography and Calcifications
The great majority of currently diagnosed cases of DCIS are nonpalpable and are detected by various radiologic techniques. Mammography is a highly sensitive diagnostic procedure for detecting DCIS.32 In 2002, it was estimated that about 1 in every 1,300 screening mammograms resulted in a diagnosis of DCIS.33 Until recently, on initial screening, 8% to 43% of mammographically detected carcinomas were intraductal.34,35,36,37,38,39,40 Twenty-five percent to 30% of nonpalpable carcinomas detected by mammography were intraductal lesions.37,41,42,43 In a series of nearly 20,000 patients, 30 of 70 carcinomas (43%) found in biopsies performed only for clustered calcifications detected by mammography were DCIS.38 Mammographically detected calcifications were found in 72% to 98% of DCIS.44,45,46,47 The proportion of DCIS was not substantially higher in subsequent mammography screening, but some investigators have described a greater frequency of small invasive tumors in later examinations.34,39
The interval between screening examinations can influence the clinical characteristics of DCIS detected by mammography.48 The size of DCIS determined by mammographic measurement was significantly smaller in women examined annually (mean, 1.69 cm; range, 0.3 to 7.7 cm) than in those examined on a biennial (mean, 2.27 cm; range, 0.4 to 10 cm) or triennial (mean, 3.49 cm; range 0.6 to 10 cm) schedule. Comedo-type (high-grade solid) DCIS was significantly more frequent in the biennial (73.7%) than in the annual (46.8%) screening group. Tumor size and nuclear grade were inversely related to the mean sizes for low-, intermediate-, and high-grade lesions determined to be 1.19, 1.85, and 2.82 cm, respectively. The frequency of microinvasion tended to increase with longer intervals between examinations, but the differences were not statistically significant.
Approximately 10% to 15% of DCIS are discovered as incidental lesions in biopsies performed for other indications, usually a palpable abnormality.32,43,46 Radiologic findings that lead to the detection of a small proportion of “incidental” DCIS are densities and asymmetric soft tissue changes, sometimes with microcalcifications in the noncarcinomatous abnormality. Calcifications alone are more likely to be the mammographic indicator of DCIS in women younger than 50 years, whereas coexistent soft tissue abnormalities are evident more often in women older than 50, a distinction that probably results from variation in overall breast density in these age groups rather than from intrinsic tumor differences.46
Relatively specific findings of DCIS on mammograms include microcalcifications of certain types and patterns, that is, the calcifications may be pleomorphic, coarse, and fine and are either clustered or linear in distribution.47 Calcifications associated with DCIS are generally described as linear “casts” or as granular on mammography (Fig. 11.1). Round or oval, well-circumscribed calcifications are less common in DCIS. Predominantly linear, granular, or mixed types of calcifications occur with approximately equal frequency in DCIS. Calcifications may be clustered, dispersed, or dispersed around clustered foci. Branching calcifications with linear patterns that outline the distribution of one or more ducts may consist of casts or granular particles. The type of calcifications is not related to age at diagnosis or to the size of the area involved mammographically.46 The level of suspicion for DCIS is a function of the character and the number of calcifications. The majority of DCIS have five or more calcifications.46
On mammograms, linear, pleomorphic calcifications are commonly seen in high-grade DCIS, and granular segmental calcifications are typical of lower grade lesions. Ducts afflicted with high-grade DCIS harbor calcifications more often than those with low-grade DCIS. In some cases of lowgrade DCIS, the majority of calcifications are in adjacent benign glands. Thus, DCIS may be smaller, larger, or equal to the extent of mammographic calcifications, and calcifications do not always “map-out” DCIS, particularly in lower grade lesions. Despite such nuances, the mammographic distribution of calcifications is commonly used as a guide to the extent of DCIS or the dimensions of the involved area. However, these measurements typically tend to underestimate the size of the lesion compared with careful histologic sampling.49 When the extents of lesions were measured mammographically and pathologically, discrepancies were found more often between the interpretations for cases that were predominantly cribriform or micropapillary than for high-grade, solid DCIS. A discrepancy of more than 20 mm was found in 44% of pure cribriform-micropapillary lesions,
in 12% of pure comedocarcinomas, and in 50% of cases with both patterns.49 In patients who undergo mastectomy, extension of DCIS to the nipple or subareolar region is more frequent with comedo than with cribriform-micropapillary DCIS.49 The likelihood of detecting multifocal DCIS radiologically and pathologically is related to the size of the lesion as determined by either procedure.44,50 Multifocality is appreciably more frequent in lesions larger than 2.0 to 2.5 cm than in smaller foci of DCIS. Carlson et al.48 reported that the mean size of multifocal DCIS (3.1 cm) was significantly greater than the size of nonmultifocal lesions (1.95 cm).
in 12% of pure comedocarcinomas, and in 50% of cases with both patterns.49 In patients who undergo mastectomy, extension of DCIS to the nipple or subareolar region is more frequent with comedo than with cribriform-micropapillary DCIS.49 The likelihood of detecting multifocal DCIS radiologically and pathologically is related to the size of the lesion as determined by either procedure.44,50 Multifocality is appreciably more frequent in lesions larger than 2.0 to 2.5 cm than in smaller foci of DCIS. Carlson et al.48 reported that the mean size of multifocal DCIS (3.1 cm) was significantly greater than the size of nonmultifocal lesions (1.95 cm).
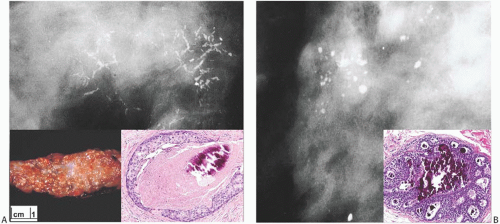 FIG. 11.1. DCIS, radiologic-pathologic correlation. A: Radiograph showing branching linear calcifications found at biopsy to be in high-grade DCIS with necrosis (“comedo” type). Inset on left: typical “comedo” appearance on cut section. Inset on right: cross section of a duct with high-grade in situ carcinoma with central necrosis and calcification. B: Clustered, rounded punctate calcifications at the site of cribriform DCIS (inset). Inset images are from cases other than those shown in the radiographs. |
The mammographic appearance of microcalcifications bears some relationship to the histologic type of the lesion, but, as noted by Stomper and Connolly,51 “there is considerable overlap, and the predominant histologic subtype cannot be predicted on the basis of the microcalcification type with a high degree of accuracy.” Predominantly linear calcifications are found significantly more often in comedocarcinomas than in cribriform, papillary, or solid types, which typically contain granular calcifications.49,51 Nonetheless, 22% of linear calcifications were associated with noncomedocarcinomas, and 47% of granular calcifications occurred in comedocarcinomas in one series.51 The presence of extensive casting-type microcalcifications occupying more than one quadrant in a mammogram was associated with highgrade DCIS, multifocal invasive duct carcinoma, and axillary nodal metastases in 33% of 12 patients who had lymph nodes examined.48
Image analysis of calcifications has had some success in discriminating between comedo and noncomedo DCIS.52 Abnormal mammograms without calcifications are more likely to call attention to DCIS of the small cell type than the large cell type, regardless of the growth pattern (solid, cribriform, or mixed) of the lesion.53 Linear calcifications are a marker of necrosis, and granular calcifications are associated with DCIS without necrosis.53 DCIS that overexpresses the human epidermal growth factor 2 (HER2) oncogene is more likely to have calcifications detected by mammography than is a HER2 negative carcinoma.54 Extent of mammographic calcifications, presence of either a radiographically or a clinically evident mass, and solid architectural type of DCIS have been demonstrated to be significantly associated with invasion on final excision.55
Unusual mammographic presentations of DCIS occur when the lesion has a configuration that suggests a benign tumor or invasive carcinoma. These patterns, reflective of associated soft tissue masses, are found in less than 10% of mammographically detected DCIS.45,56,57,58,59 In one series, 8% of DCIS were represented mammographically by stellate lesions without calcifications,59 and in another report, 3.6% of DCIS presented as stellate opacities.56 Three were pure DCIS, and four proved to be DCIS arising in benign radial sclerosing lesions or “radial scars.” Microinvasion was found in only one case, despite the radiologic appearance suggesting invasive carcinoma in all instances. At the other end of the spectrum, DCIS may be harbored by radiologically circumscribed lesions and appear to be benign.57 In addition to carcinoma arising in a fibroadenoma, these are usually examples of solid papillary DCIS or nodular foci of comedocarcinoma. Microinvasion may be present.57
Ultrasound Evaluation
Ultrasound is only uncommonly helpful in diagnosing DCIS. In general, “ductal changes” with associated microcalcifications are the most common sonographic findings in about one-third of the cases of high-grade DCIS, and an irregular hypoechoic mass with an indistinct margin is the most frequent finding in about one-third of non-high-grade DCIS cases.58
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) has proven to be an effective method for detecting DCIS, especially lesions that lack calcifications. Menell et al.60 found that MRI was more sensitive than mammography for detecting DCIS overall and for detecting multifocal DCIS. Lesion detection is based on the finding of contrast enhancement in breast parenchyma after injection of a gadolinium contrast agent compared with the preinjection image.61,62,63 Orel et al.64 described three patterns of enhancement associated with DCIS: ductal, regional, and a peripherally enhancing mass. The mean size of MRI-detected DCIS was 10 mm. Correlation of immunohistochemical studies for vascularity and MRI characteristics of the lesions suggested that tumor angiogenesis contributed to MR enhancement in one series.61 Contrast-enhanced MRI has proven to be an effective method for the detection of concurrent, unsuspected contralateral carcinoma in women with ipsilateral DCIS.65
MRI has higher sensitivity for invasive carcinoma (up to 98%) than for DCIS (sensitivity of 60% to 80%).66 On MRI, DCIS typically has a non-mass, delayed peak enhancement profile; however, this methodology has a high rate of false negatives. Gadolinium, the contrast media used in MRI, has been shown to accumulate within the intraductal space of DCIS.67
Current indications for adjunct MRI include the detection of an occult primary tumor, the examination of dense breast tissue, the presence of known BRCA mutations, and the detection of chest wall involvement.68 MRI has two main roles in the evaluation of DCIS. The first is assessing the extent of disease, and the other is early detection in breast cancer screening programs.
The sensitivity of MRI for the accurate assessment of DCIS is more than 60%, compared with approximately 55% for mammography and 45% or so for ultrasound69; MRI screening may potentially double the probability of carcinoma detection in a high-risk population compared with either mammography or ultrasound alone.68,70 Owing to the higher detection rate of otherwise occult significant disease on MRI (including so-called “elsewhere carcinoma”), there is a strong association between preoperative MRI performed in women with DCIS and mastectomy.71
Palpable DCIS
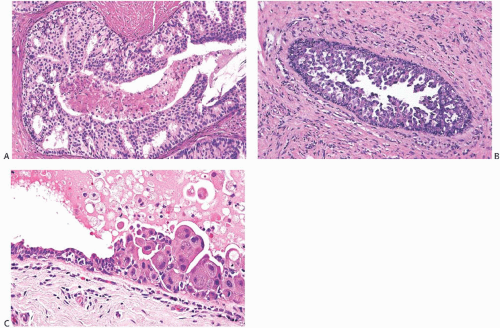
Prior to the widespread use of mammography, palpable tumors were reportedly present in 50% to 65% of women who had DCIS.72,73,74 A study comparing breast carcinomas diagnosed during 1973 to 1974 in Japan and the United States reported a higher frequency of DCIS in Japanese patients and noted that the carcinomas tended to form bulky, palpable tumors in Japanese women.75 Pandya et al.74 compared the characteristics of DCIS detected in eras prior to (1969 to 1985) and after the “intensified use of screening” (1986 to 1990) at the Lahey Clinic. The proportion of mammographically detected cases increased from 19% to 80%, whereas palpable lesions decreased from 54% to 12%. The proportion of cases presenting with duct discharge and Paget disease also decreased. Comedo DCIS was found in 7% and 38% of palpable and mammographic lesions, respectively.
Currently, DCIS is not palpable in the majority of patients with this disease.76 Negative mammograms may be reported in up to 25% of cases, with a sensitivity ranging from 56% in women younger than 40 years to 67% in the 40- to 49-year age group and 76% in those 50 years or older.32 Nonpalpable lesions are detected because of imaging findings, Paget disease, nipple discharge, or as an incidental finding in a biopsy for a concurrent palpable benign tumor41,76 (Fig. 11.2). About 25% of biopsy procedures performed for “suspicious” calcifications reveal carcinoma, largely of the intraductal type.77,78 Duct hyperplasia and sclerosing adenosis (SA) account for the majority of “significant” calcifications that do not prove to be carcinoma. Comedocarcinoma is the type most frequently detected by mammography alone, whereas micropapillary DCIS is more often found as a result of a palpable lesion or other clinical signs.76
Frozen Section Evaluation
The diagnosis of DCIS requires histologic sections of excised breast tissue. DCIS can be recognized in frozen sections (FSs), but if any difficulty is encountered, the decision should be immediately deferred to permanent sections because there is a significant risk of trimming away the lesional area if more FSs are made.79 FS is not appropriate for the diagnosis of mammographically detected, nonpalpable lesions, unless there are exceptional clinical circumstances. In one study of DCIS, 50% of the lesions were diagnosed at the time of FS, 36% were reported to be benign, 8% were deferred, 5% were diagnosed as atypical hyperplasia, and one case was diagnosed as invasive.80 Approximately 3% of biopsies reported to be benign at FS prove to contain carcinoma when paraffin sections are examined.79 Because the sampling of a biopsy is limited during surgery, approximately 20% of patients with a FS diagnosis of DCIS prove to have invasion after multiple paraffin sections of the same biopsy specimen were examined.81
The use of FS evaluation of margins in breast-conserving surgery has been shown to decrease reoperative rates; however, “technical changes in freezing breast tissue,” specifically those with “high adipose content,” is a major limitation of such analyses besides the obvious difficulty in interpretation of “atypical ducts.”82 The possibility of “skip lesions” of DCIS must be kept in mind when assessing margins of lumpectomies (and nipple margins in nipple-sparing mastectomies) by FS analyses.83
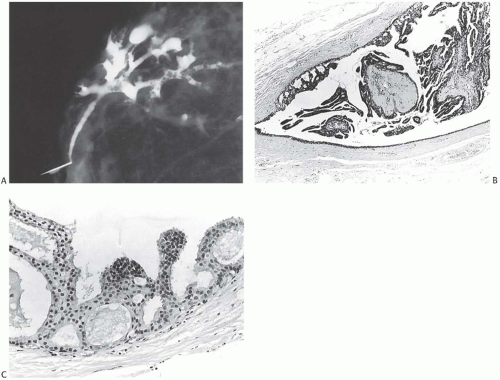 FIG. 11.2. DCIS. A: Ductogram from an 84-year-old woman with bloody nipple discharge. The cannulated lactiferous duct is seen in the lower left. Numerous defects in the white dye in ducts represent intraductal papillary lesions. B: Orderly papillary DCIS in the lumen and micropapillary carcinoma at the periphery. C: Micropapillary DCIS. |
Age at Diagnosis
DCIS occurs throughout the age range of breast carcinoma in women. The mean age at diagnosis of patients in multiple studies was between 50 and 59 years, quite similar to the mean age of women with invasive duct carcinoma.22,56,62,63 There are no significant differences in the age distributions of structural subtypes of DCIS.76
In 2012, in the United States, there is one diagnosis of DCIS for every four diagnoses of invasive breast cancer. For women 50 to 64 years of age, the incidence of DCIS has been estimated to be 88 per 100,000. The risk of DCIS is minimal in women less than 30 years of age and is low in women less than 40 years of age. Thereafter, the risk increases steadily between the ages 40 and 50, increases at a slower rate after age 50, and plateaus after age 60.15
A higher recurrence rate is associated with DCIS at a younger age, generally regarded as under 40 years of age; however, breast conservation therapy is possible in smaller, lower grade, and nonnecrotic types of DCIS in which widely negative margins have been achieved.84 Women with DCIS and a family history of ovarian carcinoma or those who had a BRCAPRO (BRCA mutation carrier prediction model) score of more than 10% had a 27% rate of BRCA1/2 mutation positivity regardless of age at diagnosis.85 BRCAPRO is a statistically derived score for assessing the probability that an individual carries a germline deleterious mutation of BRCA1 and BRCA2 genes, based on family history.
Bilaterality
Limited data are available describing the frequency of bilaterality associated with DCIS in one breast.86,87 Among 112 patients with DCIS reported by Ashikari et al.,72 16 (14%) had concurrent contralateral carcinoma, and 17 (15%) had undergone mastectomy previously for carcinoma. Westbrook and Gallager73 excluded an unstated number of patients with previous or concurrent contralateral invasive carcinoma from their study of DCIS. Subsequent contralateral biopsies obtained from 14 of the 64 women included in the report revealed DCIS in five and invasion in three others, for an overall frequency of subsequent carcinoma in the opposite breast of 12.5%. The average length of followup was not stated. Brown et al.88 found that 10% of patients with DCIS in one breast had contralateral invasive carcinoma, including three women treated previously for the contralateral lesion and one who subsequently developed contralateral carcinoma. No information about concurrent
contralateral biopsies was provided. A population-based study of cases identified in the Connecticut Tumor Registry found that 22% of 217 patients with DCIS in one breast had intraductal or invasive carcinoma in the opposite breast.22 Overall, 17% of the patients with DCIS also had a nonmammary malignant neoplasm.
contralateral biopsies was provided. A population-based study of cases identified in the Connecticut Tumor Registry found that 22% of 217 patients with DCIS in one breast had intraductal or invasive carcinoma in the opposite breast.22 Overall, 17% of the patients with DCIS also had a nonmammary malignant neoplasm.
A systematic evaluation of the contralateral breast was reported by Urban,89 who biopsied the opposite breast in 70% of his cases. Among 16 women with DCIS treated between 1966 and 1968, he found that three (19%) had had a prior contralateral mastectomy. There were no patients with simultaneous bilaterality. Ringberg et al.90 carried out bilateral mastectomy in patients with unilateral carcinoma. The contralateral breast specimens were subjected to a detailed pathologic analysis. Among 23 women with DCIS in one breast, the distribution of contralateral disease was as follows: LCIS, two cases (9%); DCIS, three cases (13%); and invasive carcinoma, two cases (9%). In another study, simultaneous contralateral mastectomy in 25 of 78 patients who had noncomedo DCIS in one breast revealed contralateral DCIS in 3 (12%).91 The type of DCIS and indications for performing the operation in these cases were not indicated. Schuh et al.92 reported that 7 of 52 (13%) patients with DCIS had previously undergone a contralateral mastectomy for carcinoma. Simultaneous bilateral carcinoma was found in 3 of the remaining 45 women (7%), including two contralateral invasive lesions and one with LCIS. Schwartz et al.93 reported that 3 of 47 patients (6%) with nonpalpable DCIS treated by mastectomy had clinically detected DCIS in the opposite breast. Silverstein et al.87 found bilateral simultaneous or metachronous carcinoma in 22 of 208 patients (11%) with pure or microinvasive DCIS, including 5 (2.4%) with bilateral intraductal lesions. Ciatto et al.32 reported contralateral carcinoma in 44 of 350 women (13%) with DCIS, including 9 (3%) with synchronous bilateral intraductal, 9 (3%) with synchronous invasive, 2 (6%) with metachronous invasive, and 5 (1.4%) with metachronous DCIS. After excluding synchronous contralateral carcinoma, Ciatto et al.32 calculated the frequency of metachronous contralateral carcinoma based on breast years at risk to be 8.5%, 5.6 times the expected risk of 1.5% for unilateral breast carcinoma in a normal population.
The occurrence of contralateral carcinoma in women with DCIS was studied in a population-based cancer registry from the state of Washington by Habel et al.94 The authors identified 1,929 women with DCIS diagnosed in one breast between 1974 and 1993. Contralateral invasive carcinoma developed at a rate twice that of the control population. When contralateral in situ carcinoma was found, it was intraductal in 78% of these patients. The detection rate for contralateral DCIS was highest in the first year after diagnosis of the ipsilateral lesion, with a relative risk (RR) compared with controls of 21.4 (95% confidence interval [CI], 11.8 to 38.7). Five years or more after ipsilateral diagnosis, the RR was 3.1 (95% CI, 1.0 to 9.8).
The frequency of subsequent invasive carcinoma in the contralateral breast of women with DCIS was 4.3% in one series, considerably less than that for patients with LCIS.95 A similar observation was recorded by Habel et al.94 who found that the RR of contralateral invasive carcinoma was 1.8 (95% CI, 1.4 to 2.4) for women with ipsilateral DCIS and 3.0 (95% CI, 1.7 to 5.1) for women with LCIS compared with a control population. The majority of deaths due to breast carcinoma recorded in patients with DCIS in one breast have been due to invasive carcinoma of the contralateral breast.32,41,72 Deaths due to contralateral invasive carcinoma were reported in 3.6%,52 1.9%,43 and 1.0%32 of cases, whereas in two of these studies deaths caused by invasive recurrence in the ipsilateral breast occurred in 2 of 140, or 1.4%,32 and 2 of 61, or 3.2%,56 of patients treated with breast conservation.
It is clear from the foregoing review that data regarding bilaterality in women with DCIS in one breast are heavily influenced by methodologic issues relating to how the information was assembled. Clinically apparent synchronous contralateral carcinoma occurs in less than 10% of patients, with at least half also being intraductal. Metachronous subsequent carcinoma occurs more frequently than initial primary DCIS in the general population, with a RR of about 2. Subsequent contralateral invasive carcinoma is responsible for the majority of breast carcinoma deaths in women with ipsilateral DCIS, ranging from 1% to nearly 4%. A small number of deaths are also attributable to ipsilateral invasive recurrences after breast conservation therapy. Contrast-enhanced MRI is an efficient method for detecting occult concurrent contralateral carcinoma in women with ipsilateral DCIS.65
GROSS PATHOLOGY
Noncomedo DCIS and nonpapillary DCIS are usually not evident grossly. Comedocarcinoma involving multiple ducts occasionally produces a firm mass (Fig. 11.3). A palpable, high-grade solid type of DCIS with necrosis (i.e., of the
comedo type) tends to be a well-defined, tan tumor with white to pale yellow flecks composed of necrotic DCIS (comedos) that extrude from the cut surface when the lesion is compressed. Abundant calcification in the lesion can impart a gritty sensation upon cutting. Although these findings are suggestive of this type of carcinoma, a similar gross appearance is found in some instances of duct ectasia and mastitis.
comedo type) tends to be a well-defined, tan tumor with white to pale yellow flecks composed of necrotic DCIS (comedos) that extrude from the cut surface when the lesion is compressed. Abundant calcification in the lesion can impart a gritty sensation upon cutting. Although these findings are suggestive of this type of carcinoma, a similar gross appearance is found in some instances of duct ectasia and mastitis.
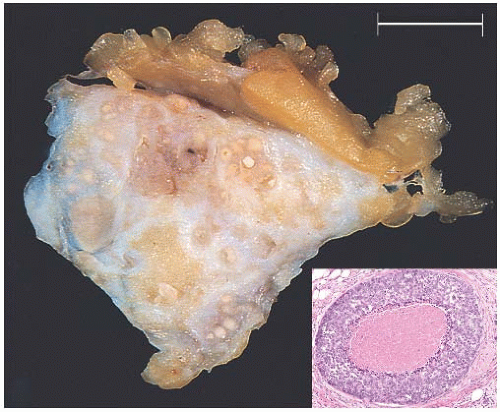 FIG. 11.3. DCIS, “comedo.” Gross biopsy specimen showing numerous round and pale yellow foci of “comedo” type necrosis. Inset: cross section of a duct with high-grade in situ carcinoma with central necrosis from another case. The latter corresponds to the “comedo” appearance on the cut section of the gross specimen. |
Most classifications of DCIS have been based on histopathologic features of the lesions, but some investigators have drawn attention to the distinction between grossly apparent and microscopic lesions. Gump et al.96 studied 70 consecutive patients treated in one institution for lesions classified as DCIS on an initial biopsy. Fifty-four (77%) had carcinomas classified as “gross” because the patient presented with a palpable tumor, nipple discharge, or Paget disease. The majority of these patients (48 of 54 or 89%) had a mass. Microscopic DCIS in 16 (23%) was nonpalpable, and it was detected by mammographic calcifications or it was an incidental finding. Invasive carcinoma was found in six (12%) surgical specimens subsequent to an initial biopsy that revealed gross DCIS, but not in the patients who had microscopic DCIS. Axillary lymph node (ALN) metastases were found in only one patient with a gross lesion, and not in any patient with microscopic DCIS.
A slightly more complex classification based on anatomic distribution was proposed by Andersen et al.97 They identified three types of growth patterns, which occurred individually or in combinations. “Microfocal” lesions involved “one or a few lobules and/or ducts” measuring up to 5 mm. “Diffuse” DCIS involved a region of 5 to 10 mm or an entire segment of the breast, and the “tumor-forming” type consisted of closely connected glandular structures that occupied an area of 60 to 70 mm, resulting in a palpable mass. Microfocal and diffuse types of DCIS were typically not palpable. A population-based review of cases revealed that 18 of 35 patients (51%) with DCIS had microfocal lesions, 13 (37%) had the diffuse type, and 4 (11%) had tumor-forming DCIS.21 No ALN metastases were found in any of the patients who had an axillary dissection.
MICROSCOPIC PATHOLOGY
General Histologic Features
The microanatomic site of origin of many DCIS appears to be in the terminal duct lobular unit (TDLU). The most convincing evidence for this conclusion comes from the subgross microdissection studies of Wellings et al.98 Expanded TDLUs sometimes resemble primary or secondary segmental ducts, but their lobular origin is suggested by an excessive number of duct structures within a low-power microscopic field and by the accompanying stroma. Recently characterized columnar cell lesions lend support to this conclusion.
Exceptions can be found to the concept of the TDLU origin of DCIS. For example, it does not readily describe DCIS limited to major central lactiferous ducts, sometimes associated with Paget disease or nipple discharge. Occasionally, random sections disclose foci of DCIS in sections of one or more segmental ducts with no apparent lobular connection even when the lesion is traced with serial sections. The relative frequency of origin from the TDLU or from larger duct structures, and the clinical significance of this distinction remain to be determined.
The microscopic classification of DCIS became the subject of heightened interest after the widespread introduction of breast conservation therapy. Interest in factors associated with the success or failure of this therapy directed attention not only to variants described on the basis of growth pattern, but also to finer cytologic details.
The spectrum of histologic patterns of DCIS in men does not differ appreciably from the appearance of the disease in women. There is a higher proportion of papillary DCIS in men, and comedo DCIS is less frequent than in women.
In standard histologic sections, DCIS is confined within the lumens of ducts and lobules involved in the process. When studied by immunohistochemistry (IHC) for laminin or type IV collagen, basement membranes in DCIS appear intact or focally discontinuous.99,100,101 The presence or absence of mitotic figures is not a definitive feature in the diagnosis of DCIS, because mitoses may also be found rarely in normal and hyperplastic epithelium. However, the finding of one or more mitoses per 10 high-power fields (HPFs) suggests DCIS.
Myoepithelium in DCIS
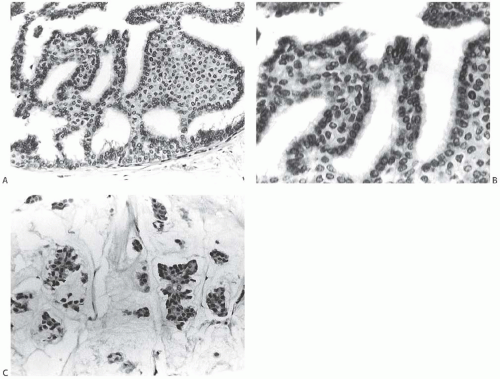
Myoepithelial cells are often retained but attenuated in DCIS, and they are occasionally hyperplastic at the periphery of the duct (Fig. 11.4). Myoepithelial cells do not generally accompany the neoplastic epithelial proliferation within the duct lumen in DCIS except for certain types of carcinoma arising in a papilloma or in solid papillary DCIS.
Experimental evidence suggests that myoepithelial cells may have a paracrine tumor suppressor effect on DCIS, acting to inhibit invasion.102 Tumor suppression capabilities of myoepithelial cells include inhibition of invasion103 and of angiogenesis.104 In vitro, myoepithelial cells have been shown to have the capacity to inhibit breast carcinoma cell growth and to induce apoptosis.105 These tumor-inhibiting properties have been attributed in part to the expression of maspin, a protease inhibitor, by myoepithelial cells.106 Other tumor suppressor genes expressed by myoepithelial cells include cytokeratin 5 (CK5), smooth muscle actin (SMA), and caveolin-1.107
The functional activity of myoepithelial cells in in situ carcinoma is significantly changed compared with normal epithelium. These alterations include overexpression of chemokines CXCL12 and CXCL14, which bind to and enhance the invasiveness of carcinoma cells.108 In particular, CXCL12 and its receptor CXCR4 appear to promote breast carcinoma cell growth and metastases.109,110
Cell Types in DCIS
The range of subtle differences in cell type found in DCIS usually engenders little comment, but certain distinct variants have been identified and described by specific names. Signet
ring cells, usually associated with lobular carcinoma, also occur in DCIS, most often in the papillary and cribriform types (Fig. 11.5). The presence of signet ring cells with cytoplasmic mucin demonstrated with the mucicarmine, periodic acid-Schiff (PAS), or Alcian blue stains is strong evidence for a diagnosis of DCIS. These cells are present only very rarely in hyperplastic duct lesions. Signet ring cells have eccentric nuclei that are often along the nuclear border, which abuts on the cytoplasmic mucin vacuole. A minute droplet of secretion may be apparent in the vacuole. Intracytoplasmic mucin sometimes imparts a diffuse pale blue color to the cytoplasm of carcinoma cells without forming distinct vacuoles. Nonspecific clear holes in the cytoplasm can be mistaken for signet ring vacuoles. These cytoplasmic defects, sometimes the site of glycogen accumulation, are not reactive with stains for mucin; they usually do not indent the nucleus, and there is ordinarily no secretion evident in the lumen.
ring cells, usually associated with lobular carcinoma, also occur in DCIS, most often in the papillary and cribriform types (Fig. 11.5). The presence of signet ring cells with cytoplasmic mucin demonstrated with the mucicarmine, periodic acid-Schiff (PAS), or Alcian blue stains is strong evidence for a diagnosis of DCIS. These cells are present only very rarely in hyperplastic duct lesions. Signet ring cells have eccentric nuclei that are often along the nuclear border, which abuts on the cytoplasmic mucin vacuole. A minute droplet of secretion may be apparent in the vacuole. Intracytoplasmic mucin sometimes imparts a diffuse pale blue color to the cytoplasm of carcinoma cells without forming distinct vacuoles. Nonspecific clear holes in the cytoplasm can be mistaken for signet ring vacuoles. These cytoplasmic defects, sometimes the site of glycogen accumulation, are not reactive with stains for mucin; they usually do not indent the nucleus, and there is ordinarily no secretion evident in the lumen.
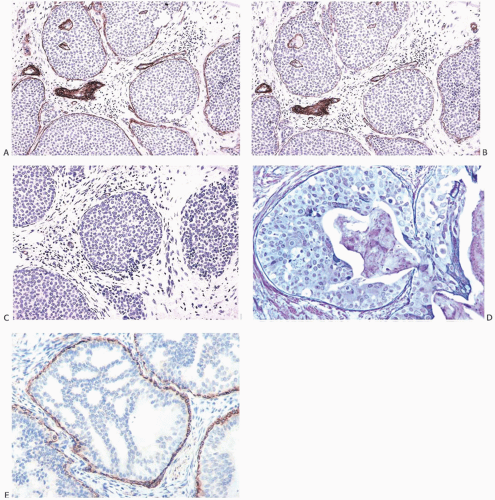 FIG. 11.4. DCIS with basal lamina and myoepithelial cells. A-C are from the same specimen. A: The basal lamina is highlighted by the immunostain for type IV collagen. Reactivity is also present around small blood vessels, including vessels in the upper two ducts. B: Laminin reactivity shown here has the same distribution as type IV collagen. C: There is no reactivity for SMA indicating the absence of myoepithelium in this DCIS. D: Basement membrane is highlighted by the reticulin stain. E: In this example of cribriform DCIS, myoepithelial cells display reactivity for the SMA. |
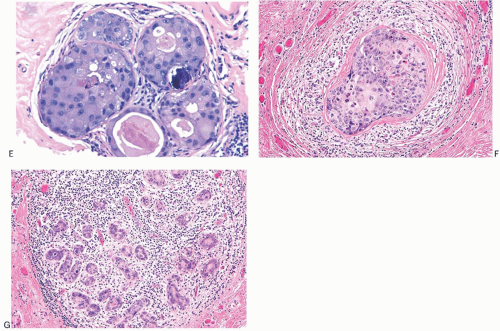
Apocrine cytology is encountered in all of the structural types of DCIS (Fig. 11.6). Apocrine DCIS cells have abundant cytoplasm that ranges from granular and eosinophilic to vacuolated or clear. There is variable nuclear pleomorphism, sometimes manifested by prominent nucleoli. A more complete discussion of apocrine carcinoma can be found in Chapter 19.
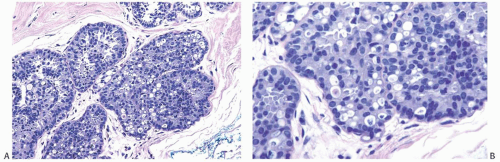 FIG. 11.5. DCIS with signet ring cells. A,B: Many of the tumor cells have cytoplasmic vacuoles that contain condensed secretion. |
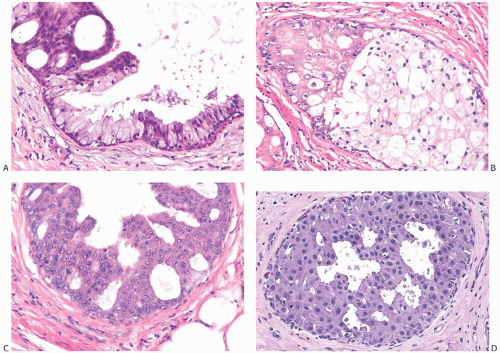 FIG. 11.6. DCIS, apocrine. A: Micropapillary carcinoma, partly clear cell type. B: Solid carcinoma, partly clear cell type. C: Cribriform carcinoma with high nuclear grade. D: Cribriform carcinoma with low and intermediate nuclear grade. E: Apocrine carcinoma in enlarged lobular glands with calcification. F-G: Apocrine carcinoma of solid type with high nuclear grade and lobular extension (G). Note the periductal (F) and intralobular (G) lymphocytic infiltrate. |
Clear cell DCIS is a poorly defined variant typically encountered with solid and comedo patterns (Fig. 11.7). Some clear cell DCIS are composed of cells with an arrangement described as “mosaic” because of the appearance created by sharply defined cell borders (Fig. 11.7). A subset of lesions classified under this heading includes forms of apocrine carcinoma. The presence of a monomorphic clear cell population is highly suggestive of DCIS. Occasionally, clear cell DCIS are strongly mucicarmine positive. Other clear cell lesions are probably the in situ form of lipid-rich or glycogenrich carcinomas discussed in separate chapters.
Spindle cell DCIS may express neuroendocrine markers such as chromogranin, synaptophysin, and neuron-specific enolase.111,112 The swirling growth pattern of cells in spindle cell DCIS mimics “streaming,” which is characteristically found in usual duct hyperplasia. Spindle cell DCIS often coexists with cribriform DCIS.
Small cell DCIS is extremely uncommon. The growth patterns are typically cribriform and solid or a mixture of these forms. When present by itself, the solid pattern of small cell DCIS can be distinguished from LCIS with the E-cadherin immunostain that demonstrates membrane reactivity in DCIS. E-cadherin staining is absent or fragmented and weak in LCIS. “Neuroendocrine” DCIS is a less aggressive variant of small cell carcinoma (SCC) that is typically characterized by solid growth and spindle cell with fine granular cytoplasm, immunoreactivity for neuroendocrine markers, and a lower proliferation rate than SCC.112
The cellular composition of DCIS is typically monomorphic. This term has been applied especially to cribriform, solid, and micropapillary carcinomas. In this context, monomorphic means that there is overall homogeneity in the cytologic appearance of the DCIS cells—although there may be minor variation among cells in terms of amount of cytoplasm, nuclear size, etc. Variability in these parameters derives in part from differences in the plane in which they are sectioned. Cell and nuclear shape may be altered by the presence or absence of crowding in one or another part of the duct. The presence of a myoepithelial cell layer is not a consideration in judging whether a ductal proliferation is monomorphic.
Dimorphic variants of DCIS consisting of two distinctly different populations of cells are unusual. The majority of dimorphic DCIS are papillary carcinomas (see Chapter 14). A dimorphic papillary DCIS with a small invasive component of mucinous carcinoma is illustrated in Figure 11.8.
DCIS exhibits considerable tumoral heterogeneity, and in a given patient can have more than a single microscopic structural, cytologic, or immunocytochemical phenotype.76,113,114 Mixed histologic patterns are found in
approximately 50% of cases. Whereas some structural combinations, such as papillary- or micropapillary-cribriform and solid-comedo, occur relatively more often than others, there is considerable heterogeneity with respect to growth patterns.115 The probability of structural variability increases with the size of the lesion. Needle core biopsy samples may not be representative of the diverse growth patterns in a single case. The histologic diagnosis of DCIS should list the structural types in order of decreasing prominence, placing the dominant pattern first.
approximately 50% of cases. Whereas some structural combinations, such as papillary- or micropapillary-cribriform and solid-comedo, occur relatively more often than others, there is considerable heterogeneity with respect to growth patterns.115 The probability of structural variability increases with the size of the lesion. Needle core biopsy samples may not be representative of the diverse growth patterns in a single case. The histologic diagnosis of DCIS should list the structural types in order of decreasing prominence, placing the dominant pattern first.
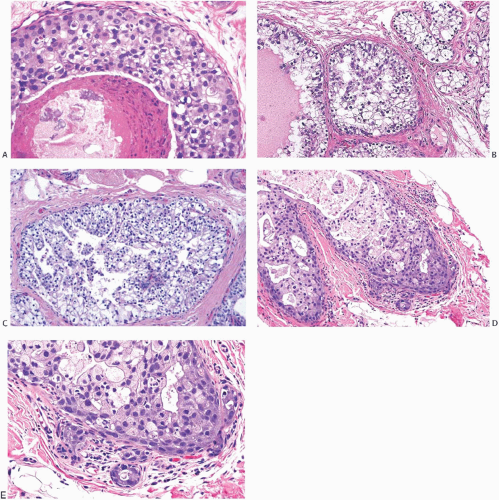
Cytologic features, especially at the nuclear level, tend to be more homogeneous than the growth pattern in a given case. Some combinations of growth patterns and cytologic appearances occur more frequently, such as classic comedo DCIS composed of poorly differentiated pleomorphic cells or the low nuclear grade typically present in micropapillary DCIS. Heterogeneity is illustrated by lesions composed of small, cytologically low-grade nuclei growing in a solid pattern or by high-grade nuclei found in some examples of micropapillary DCIS (Fig. 11.9). The presence of two or more structural patterns that have different cytologic features is particularly unusual (Fig. 11.10). Classification schemes developed to take cognizance of the heterogeneous distribution of nuclear grade and necrosis across the spectrum of structural patterns are considered subsequent to a discussion of the current conventional structural classification.
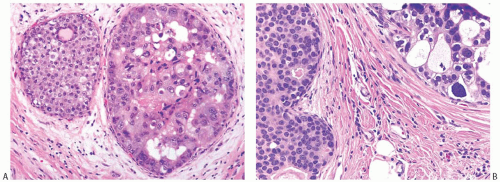 FIG. 11.10. DCIS. A: The smaller duct contains solid carcinoma with low nuclear grade and the larger duct contains cribriform carcinoma with necrosis and high nuclear grade. B: The duct on left contains solid DCIS with intermediate-grade nuclei, and the duct on the right shows cribriform DCIS with relatively higher grade nuclei and calcification. |
Structural Classification of DCIS
Micropapillary DCIS consists of ducts lined by a layer of neoplastic cells giving rise at intervals to papillary fronds or arcuate formations protruding into the duct lumen. When micropapillae are inconspicuous or absent, this type of DCIS has been described as flat or “clinging” because the neoplastic epithelium seems to hug the basement membrane (Fig. 11.11).116 The papillae are variable in appearance, ranging from short bumps or mounds to long slender processes (Fig. 11.12). The papillae lack a fibrovascular core and are composed of cytologically homogeneous carcinoma cells. Lesions in which the carcinomatous epithelium is supported by fibrovascular stroma should be classified as papillary carcinomas, even if the growth pattern is predominantly micropapillary (Fig. 11.13). Arcuate structures, commonly referred to as Roman bridge (or aqueduct) arches, occur when microlumens are formed under adjacent coalescent fronds or within a mound of neoplastic cells. These fenestrations resemble the lumens formed in cribriform DCIS (Fig. 11.14). In conjunction with micropapillae, these arches are a feature of micropapillary DCIS and do not warrant a diagnosis of cribriform DCIS. In some situations, the micropapillary and cribriform patterns merge (Fig. 11.15). Some samples of micropapillary DCIS develop complex, frondforming structures without evolving into cribriform growth (Fig. 11.16).
The appearance of the micropapillary fronds varies somewhat with the plane of individual histologic sections. Whereas some micropapillae are cut perpendicular to their long axis, others are seen sectioned tangentially or transversely, resulting in irregular nests of seemingly detached cell clusters in the duct lumen (Fig. 11.16). Ducts with low nuclear grade micropapillary DCIS are usually relatively free of cellular debris or inflammatory cells. Calcifications that are granular, crystalline, or laminated occur particularly when carcinoma arises in a background of columnar cell hyperplasia (CCH) (Fig. 11.11).
In micropapillary DCIS, the normal epithelial layer of the duct is replaced by a single population of neoplastic cells. In any given case, the appearance of the carcinoma cells is relatively homogeneous, but cytologic heterogeneity can occur between individual cases. Most often, micropapillary DCIS is composed of cytologically low-grade homogeneous cells with a high nuclear-to-cytoplasmic ratio and dense, hyperchromatic nuclei (Figs. 11.12, 11.14, and 11.15). The nuclei typically vary little in size, and chromatin density is consistent between cells at the base and tip of micropapillae. Nuclei may be slightly smaller and darker at the surface, but marked disparity in these characteristics is a feature of micropapillary hyperplasia (see Chapter 9). At the margin of the duct, between papillary and arcuate structures, the neoplastic cells are typically arranged in a layer that rarely exceeds three cells in depth. The nuclei of the cells in the epithelium between micropapillae are usually unevenly distributed in relation to the basement membrane (Figs. 11.12, 11.14, and 11.16). Persistent nonneoplastic epithelium between micropapillae is a feature of micropapillary hyperplasia rather than of micropapillary carcinoma. Mitoses are rarely present in low-grade micropapillary DCIS. The carcinoma cells tend to be so crowded and overlapping that their individual borders and cytoplasm cannot be identified. Occasionally, the cells have slightly more abundant cytoplasm, with apocrine-type protrusions at the luminal border. In one variant of this cell type, the nuclei of the tumor cells are contained in cytoplasmic blebs that are extruded into the duct lumen. Low-grade micropapillary DCIS can be found near some tubular carcinomas. These patients often have multifocal CCH with atypia and may also have LCIS
(see Chapter 9). Squamous metaplasia can be encountered in micropapillary DCIS (Fig. 11.17). Clear cell micropapillary DCIS is uncommon (Fig. 11.18).
(see Chapter 9). Squamous metaplasia can be encountered in micropapillary DCIS (Fig. 11.17). Clear cell micropapillary DCIS is uncommon (Fig. 11.18).
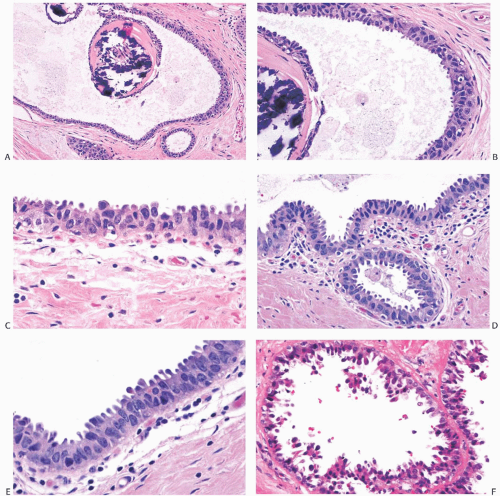 FIG. 11.11. DCIS, flat (“clinging”) micropapillary type. A: The papillary structures in this cystically dilated duct contain fractured calcifications of the ossifying type typically associated with columnar cell lesions. B: Carcinoma cells with pleomorphic nuclei and a disorderly distribution line the duct and overlie the calcification. C: The flat carcinomatous epithelium displays apical apocrinetype cytoplasmic “snouts.” D-F: Other examples of flat, “clinging” DCIS. |
A minority of micropapillary carcinomas are composed of cells with intermediate- or high- (poorly differentiated) grade cytologic characteristics (Figs. 11.9, 11.16, and 11.19). Cells forming this type of carcinoma differ from those in the conventional micropapillary lesions by being larger, with more abundant cytoplasm. Nuclei are also correspondingly larger, and nucleoli may be apparent. Mitoses can be found in this epithelium, and the cells often have a distinctly apocrine appearance. The cytologically high-grade form of
micropapillary DCIS is more likely to have calcifications than the low-grade variant, and necrotic cellular debris may be found in the duct lumen.
micropapillary DCIS is more likely to have calcifications than the low-grade variant, and necrotic cellular debris may be found in the duct lumen.
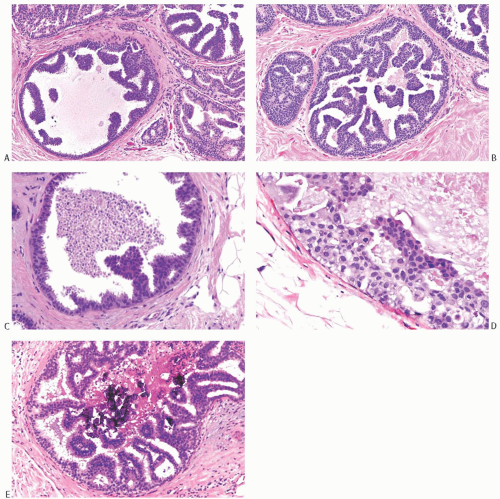 FIG. 11.12. DCIS, micropapillary. A,B: Mixed flat and micropapillary carcinoma. C: Micropapillary fronds and central necrosis are shown. D,E: Arcuate micropapillary fronds extend into duct lumens. Dense calcific deposits are present in (E). |
In an interinstitutional study, it was found that high nuclear grade micropapillary DCIS more frequently overexpressed HER2, had a higher proliferation index, displayed necrosis and microinvasion, and was more extensive than those of low- and intermediate-grade nuclei.117 Furthermore, in the same series, high nuclear grade was found to be the only parameter associated with elevated risk of local recurrence after breast-conserving surgery for micropapillary DCIS.
Two subtypes of micropapillary carcinoma have been given specific designations. Cystic hypersecretory DCIS is discussed in Chapter 24. The term flat micropapillary carcinoma (so-called “clinging” carcinoma) refers to DCIS with the cytologic appearance of the micropapillary lesion that is lacking in fully developed epithelial fronds (Fig. 11.11). Lesions composed entirely of flat micropapillary DCIS are very uncommon, and more often one or more epithelial fronds or bridges are present. In the absence of calcification or necrosis, flat micropapillary DCIS is easily overlooked microscopically. This type of DCIS is most often found in a background of CCH, which is encountered mainly in
women 35 to 55 years of age. The lesions are typically multifocal or multicentric and can be bilateral. Flat micropapillary (“clinging”) DCIS should be diagnosed whenever a flat epithelial proliferative process shows relatively small- to medium-sized cells with high-grade round-to-oval nuclei with speckled chromatin. Such neoplastic cells are generally uniform, with centrally placed nuclei within which nucleoli are inconspicuous. So-called “flat epithelial atypia” (in most cases synonymous with atypical CCH) and low-grade DCIS of the breast have been shown to share highly homologous molecular and genomic profiles118; however, such data can be interpreted as being reflective of the difficulty in distinguishing between the two entities, morphologically as well as by molecular criteria, and the need to be conservative in the diagnosis of low-grade DCIS.
women 35 to 55 years of age. The lesions are typically multifocal or multicentric and can be bilateral. Flat micropapillary (“clinging”) DCIS should be diagnosed whenever a flat epithelial proliferative process shows relatively small- to medium-sized cells with high-grade round-to-oval nuclei with speckled chromatin. Such neoplastic cells are generally uniform, with centrally placed nuclei within which nucleoli are inconspicuous. So-called “flat epithelial atypia” (in most cases synonymous with atypical CCH) and low-grade DCIS of the breast have been shown to share highly homologous molecular and genomic profiles118; however, such data can be interpreted as being reflective of the difficulty in distinguishing between the two entities, morphologically as well as by molecular criteria, and the need to be conservative in the diagnosis of low-grade DCIS.
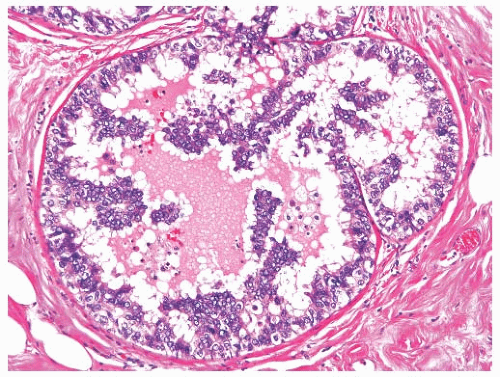 FIG. 11.13. DCIS, micropapillary. Some epithelial fronds have delicate fibrovascular centers. |
Calcifications with distinctive crystalline, ossifying, and laminated appearances tend to occur in CCH, leading to mammographic detection. Patients with CCH may have tubular carcinoma, LCIS, and invasive lobular carcinoma, as well as micropapillary DCIS.