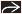Ductal Carcinoma In Situ, Paget Disease
Key Facts
Terminology
Mammary Paget disease (MPD)
Uncommon clinical presentation of breast cancer involving nipple
Clinical Issues
Occurs in 1-2% of women with breast cancer
Clinical appearance: Scaling and redness in affected area
Patients with palpable mass due to invasive carcinoma have worse prognosis
Image Findings
Mammogram may be negative or show changes related to tumor in underlying breast tissue
MR may be useful in detection of occult neoplastic disease in underlying breast tissue
Microscopic Pathology
Adenocarcinoma cells, single and in clusters, present within keratinizing epidermis of nipple
Tumor cells extend from lactiferous sinuses to nipple skin without crossing basement membrane
Immunohistochemistry panel helpful in distinguishing MPD from melanoma and squamous cell carcinoma
Top Differential Diagnoses
Carcinoma directly invading nipple skin
Toker cell hyperplasia
Squamous cell carcinoma in situ/Bowen disease
Melanoma
Clear cell change in keratinocytes
TERMINOLOGY
Abbreviations
Ductal carcinoma in situ (DCIS)
Mammary Paget disease (MPD)
Synonyms
Paget disease of nipple
DCIS involving nipple skin
Definitions
Uncommon clinical presentation of breast cancer involving nipple
MPD described by Sir James Paget (1874)
“An eruption on the nipple and areola … with characteristics of ordinary chronic eczema”
Paget linked skin changes with later development of cancer in underlying breast
MPD was later shown to be due to spread of carcinoma cells into nipple epidermis from lactiferous sinuses
Pagetoid spread is presence of tumor cells between basement membrane and overlying layer of normal cells
In nipple skin, pagetoid spread is almost always due to DCIS with overlying squamous cells
In ducts and lobules, pagetoid spread is most commonly seen with LCIS with overlying luminal cells
Unlike LCIS, DCIS typically overgrows or pushes aside overlying luminal cells and fills ducts and lobules
ETIOLOGY/PATHOGENESIS
Pathogenesis of Paget Disease
Remains debatable
In most cases, Paget cells likely originate from DCIS involving lactiferous sinuses of nipple
Supported by finding of DCIS deeper in breast identical to Paget cells in almost all cases
Very rarely, Paget cells may be derived from precursor cells (Toker cells) present within nipple epidermis
In such cases, cancer may not be present in underlying breast
Motility factor (heregulin-a) secreted by epidermal keratinocytes may attract Paget cells within nipple epidermis
Heregulin-a binds to HER2 family receptors that are overexpressed by Paget cells
Tumor cells disrupt normal tight junctions between keratinocytes
Extracellular fluid can escape through skin, and this produces characteristic scale crust
Diagnosis can sometimes be made using cytologic preparations of skin scrapings
CLINICAL ISSUES
Presentation
Skin lesions
Occur in 1-2% of women with breast cancer
May be limited to nipple or extend to areola
Scaling and redness in affected area
Pain and itching are frequent symptoms
Ulceration or serosanguineous/bloody discharge may be present in more advanced cases
Delay in diagnosis of MPD may be related to initial diagnosis of eczema or inflammatory skin disorder
Underlying breast cancer found in > 95% of cases (invasive &/or DCIS)
No age predilection seen
No clinical or epidemiologic factors have been described that predispose to development of MPD
Up to 1/2 of patients have palpable tumor on affected side
Most of these patients have associated invasive carcinoma
In very rare cases, invasion occurs directly from nipple skin into dermis
Majority of cases of MPD diagnosed microscopically are not detected clinically
Focal nipple involvement is insufficient to produce clinically detected symptoms
Treatment
Surgical approaches
Determined by presence and extent of underlying breast cancer
Due to nipple involvement, mastectomy is often performed
Adjuvant therapy
Features of associated breast cancer, including grade and extent, dictate need for and type of adjuvant therapy
Prognosis
Determined by presence and extent of underlying breast cancer
For MPD associated with underlying DCIS only, survival approaches 100% at 10 years after mastectomy
10-year survival for node-negative patients with palpable invasive carcinoma is 70%
IMAGE FINDINGS
Mammographic Findings
In early cases, imaging findings may be absent
Skin thickening is typical finding in advanced cases
MPD associated with mammographic density or nipple retraction is more likely to have areas of invasion
Calcifications may be associated with underlying DCIS
MR Findings
Typically shows abnormal nipple enhancement &/or ill-defined, thickened nipple-areolar complex
MR may be useful in detection of occult neoplastic disease in underlying breast tissue
In setting of negative mammography, MR can facilitate treatment planning for patients with MPD
MACROSCOPIC FEATURES
General Features
Gross changes reflect features seen clinically
Frequently, erythematous appearance with crusting of skin
Skin may show ulceration
However, skin preparation prior to surgery often removes gross scaling crust in surgical specimens
Palpable mass lesion may be present in underlying breast parenchyma
MICROSCOPIC PATHOLOGY
Histologic Features
Adenocarcinoma cells, single and in clusters, present within keratinizing epidermis of nipple
Clusters of Paget cells more common in basal portion of epidermis
Tumor cells extend from lactiferous sinuses to overlying skin without crossing basement membrane
Therefore, Paget disease can occur in absence of stromal invasion
Paget cells are large and atypical in appearance, stand out from surrounding keratinocytes
Enlarged pleomorphic nuclei, which tend to show prominent nucleoli
Abundant pale or eosinophilic cytoplasm
Cytoplasm may contain diastase-resistant PAS positive globules consistent with mucin
Moderate to intense lichenoid lymphocytic infiltrates typically seen in underlying superficial dermis
May obscure diagnosis, should not be mistaken for dermatitis
Varying degrees of hyperplasia and hyperkeratosis of associated epidermis
Inflammation, hyperplasia, and hyperkeratosis responsible for clinical appearance of lesion
May be associated with ulceration of epidermis
Associated ductal carcinoma (with or without invasion) usually found in underlying breast
Associated DCIS is typically high grade with solid or comedo pattern
ANCILLARY TESTS
Immunohistochemistry
Panel of immunohistochemistry stains is helpful in establishing glandular origin of Paget cells
DIFFERENTIAL DIAGNOSIS
Carcinoma Directly Invading Nipple Skin
Subareolar tumor with infiltration of superficial dermal collagen and overlying epidermis
Skin ulceration is usually present
Invasive carcinomas may involve dermis in horizontal pattern for 1-2 mm
Toker Cells and Toker Cell Hyperplasia
Epidermally located breast ductal epithelium
Most common near duct orifices
Present in at least 70% of normal nipples when detected with immunohistochemical studies
Benign appearance, bland nuclei, inconspicuous nucleoli
Toker cells share some IHC features with Paget cells
Cells are positive for CK7, CAM5.2, and EMA but are usually negative for mucin, CEA, and HER2
In Toker cell hyperplasia, cells are numerous and may show some nuclear atypia
Usually incidental finding; it would be highly unusual to be associated with clinical findings
Squamous Cell Carcinoma In Situ/Bowen Disease
Extensive replacement of nipple epidermis by Paget cells can mimic squamous cell carcinoma in situ
Squamous cell carcinoma in situ is not associated with underlying breast cancer
Usually positive for high molecular weight cytokeratins (CK5/6, CK20) and negative for mucin and HER2
Melanoma
Melanoma cells show nesting at dermo-epidermal junction
“Buck shot” spread in overlying epidermis
Paget cells may take up melanin pigment released by epidermal cells or melanocytes, simulating melanoma
Immunohistochemical staining pattern is helpful for confirming diagnosis
Clear Cell Change in Keratinocytes
Clear cell change, benign cytology, bland nuclei
More frequently seen in basal and mid layers of epidermis
SELECTED REFERENCES
1. Lester T et al: Different panels of markers should be used to predict mammary Paget’s disease associated with in situ or invasive ductal carcinoma of the breast. Ann Clin Lab Sci. 39(1):17-24, 2009
2. Park S et al: Useful immunohistochemical markers for distinguishing Paget cells from Toker cells. Pathology. 41(7):640-4, 2009
3. Di Tommaso L et al: Toker cells of the breast. Morphological and immunohistochemical characterization of 40 cases. Hum Pathol. 39(9):1295-300, 2008
4. Liegl B et al: Mammary and extramammary Paget’s disease: an immunohistochemical study of 83 cases. Histopathology. 50(4):439-47, 2007