Ductal Carcinoma In Situ
Key Facts
Etiology/Pathogenesis
Same molecular subtypes recognized in invasive carcinoma are also seen in DCIS
Transition to invasion is poorly understood
May involve loss of function of normal myoepithelial and stromal cell rather than gain of function of tumor cells
Clinical Issues
Incidence
Without mammographic screening: < 5% of carcinomas
With screening: 20-30% of carcinomas
Clinical presentations
Mammographic calcifications (85%)
Palpable mass or radiologic density (10%)
Nipple discharge (5%)
Natural history
If untreated, ˜ 1% of patients per year develop invasive carcinoma at same site
With treatment, rate of recurrence is < 10%
Some recurrences are biologically related to DCIS, and some are new primary carcinomas
Survival rate for women with DCIS is greater than that for women without breast cancer
Top Differential Diagnoses
Lobular carcinoma in situ
Atypical ductal hyperplasia
Invasive carcinoma
Lymph-vascular invasion
Collagenous spherulosis
Gynecomastoid hyperplasia
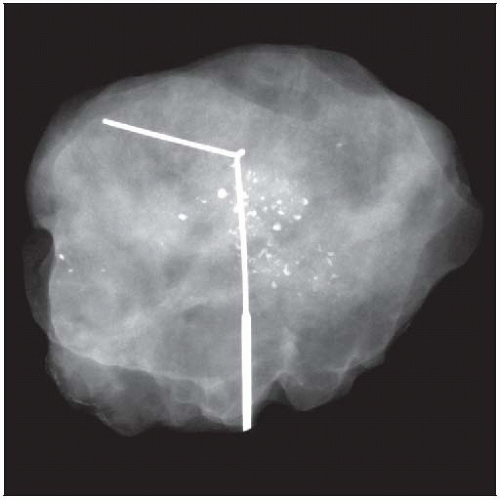 DCIS is most commonly diagnosed when clustered calcifications are detected by screening mammography. The calcifications are typically numerous and variable in size and shape. |
TERMINOLOGY
Abbreviations
Ductal carcinoma in situ (DCIS)
Synonyms
Intraductal carcinoma (IDC)
Not recommended since the abbreviation can be confused with invasive ductal carcinoma
Ductal intraepithelial neoplasia (DIN)
Only some subtypes of DIN are equivalent to DCIS
Definitions
Clonal proliferation of epithelial cells confined to ducts and lobules with a cohesive pattern and typically E-cadherin positive
ETIOLOGY/PATHOGENESIS
Biology of DCIS
Same molecular subtypes recognized in invasive carcinoma are also seen in DCIS
Luminal A (ER positive, HER2 negative): ˜ 70%
“Luminal B HER2 positive” (ER positive, HER2 positive): ˜ 10-20%
HER2 (ER negative, HER2 positive): ˜ 20-30%
Triple negative (ER/PR/HER2 negative): 5-10%
HER2 subtype is slightly more frequent and triple negative subtype less frequent in DCIS as compared to invasive carcinoma
More biologic heterogeneity is seen in DCIS than in invasive carcinoma
No specific differences in genetic alterations or gene expression have been found specific to invasive carcinoma that are not found in DCIS
Changes necessary for transition to invasive carcinoma are not yet understood
Not all DCIS progresses to invasive carcinoma
Possible that invasion occurs due to loss of function of normal myoepithelial and stromal cells, rather than gain of function by DCIS
CLINICAL ISSUES
Epidemiology
Incidence
DCIS comprises < 5% of breast carcinomas in populations without mammographic screening
With screening, 20-30% of carcinomas are detected as DCIS
Incidence of DCIS increased after introduction of screening (1980s)
Majority of DCIS is diagnosed due to formation of calcifications detectable by mammography
Presentation
Mammographic calcifications (85%)
Associated with either necrosis or secretory material in lumens
Palpable mass or radiologic density (10%)
Usually associated with extensive high-grade DCIS with periductal stromal fibrosis
Nipple discharge (5%)
Discharge is spontaneous and unilateral
Extensive micropapillary and papillary DCIS are the most common types
Paget disease of nipple (< 1%)
Patients present with eczematous scale crust of 1 nipple
DCIS traverses lactiferous ducts onto nipple skin without crossing contiguous basement membranes
DCIS is generally high grade, and most overexpress HER2
Treatment
Surgery is used to completely remove ductal system involved by DCIS
Risk of recurrence is lower if DCIS is ≥ 0.2 cm from margins
Risk of recurrence after mastectomy is < 5%
Radiation therapy reduces recurrence by ˜ 50%
Tamoxifen also reduces recurrence by ˜ 50%
Benefit may be greater, or restricted, to ER-positive DCIS
Data supporting this finding is only published in an abstract of a subset analysis of NSABP B-24
Possibility of small effect for ER-negative DCIS not excluded due to small number of cases
Prognosis
If untreated, approximately 1% of patients per year develop invasive carcinoma at same site
At 20-30 years, majority of patients remain disease free
If treated with complete excision with negative margins and possible addition of radiation therapy &/or hormonal therapy, risk of recurrence is < 10%
Approximately 1/2 of recurrences are DCIS and 1/2 are invasive carcinoma
Lymph node metastases are very rare in cases of DCIS that have been completely examined microscopically
When present, usually consist of isolated tumor cells that have not been shown to have an effect on prognosis
If a macrometastasis is present, there is usually an undetected area of invasive carcinoma
Sentinel node biopsy may be performed in patients with large areas of DCIS that are difficult to completely sample
If treated with mastectomy, risk of recurrence is < 5%
Local recurrence may be due to breast tissue left behind in chest wall
Nodal or distant recurrence may be due to undetected invasive carcinoma at time of surgery due to extensive DCIS
Risk of dying of breast cancer after recurrence in breast is very low; most cancers are detected early and are small and node negative
Survival rate for women with DCIS is greater than that for women without breast cancer
Majority of women with DCIS have access to medical care, which is not true of women in general population
Pathologic prognostic factors can predict likelihood of ipsilateral recurrence
Nuclear grade
Necrosis
Extent: Volume of breast tissue occupied by DCIS
Margin width
Some recurrences are true recurrences (related to the DCIS), and some are new primary carcinomas
Risk of contralateral invasive carcinoma is approximately 1/2 that of ipsilateral invasive carcinoma
Suggests that 1/2 of ipsilateral invasive carcinomas may be due to new primary carcinomas
May explain why surgery with wide margins without radiation therapy does not eliminate possibility of subsequent cancer in same breast
Core Needle Biopsy
DCIS is usually detected on core needle biopsies for calcifications
Presence of microinvasion may influence a decision to perform a lymph node biopsy and should be documented if present
Subsequent excisions infrequently reveal invasive carcinoma if large bore vacuum-assisted biopsies are performed and targeted lesion was calcifications and not a mass
IMAGE FINDINGS
Mammographic Findings
Calcifications are most common presenting feature for DCIS
Features correlated with DCIS
Clustered pattern
Linear and branching pattern
Large number of calcifications
Small size (large calcifications are more likely to be associated with benign lesions)
Irregular or pleomorphic shape
Increasing over time
20-30% of biopsies for suspicious calcifications will reveal DCIS on excision
Extent of DCIS is generally greater than that suggested by distribution of calcifications
Grade of DCIS is not reliably predicted by shape or number of calcifications
However, linear and branching calcifications are often associated with comedo DCIS
Calcifications without a mass are rarely associated with invasive carcinoma
In majority of cases, if invasive carcinoma is present, then the calcifications are associated with DCIS
In unusual cases, calcifications are associated with secretions in tubules or with necrosis in invasive carcinoma
Invasive carcinoma is generally small (< 1 cm)
DCIS sometimes forms a circumscribed mass
Localized area of DCIS with surrounding fibrotic stromal response can form a rounded or lobulated mass
DCIS involving a fibroadenoma can be detected as a circumscribed mass
Solid, solid papillary, or papillary DCIS can form masses
MR Findings
Majority of cases of DCIS are associated with enhancement
Although sensitive, findings are not specific enough for MR to be used for screening general population
Most common pattern is linear clumped enhancement
DCIS is typically surrounded by collarette of small capillaries
Associated increased blood flow is detected by MR
MACROSCOPIC FEATURES
General Features
Most cases of DCIS cannot be seen or palpated grossly
Cases of high-grade DCIS (typically comedo type) often have associated stromal response
Masses are usually firm but not hard
Borders are ill defined as opposed to distinct edge of invasive carcinoma
Color may be gray and texture gritty
Comedo-type necrosis can be seen grossly as minute extruded plugs of necrotic cells when tissue is gently squeezed
MICROSCOPIC PATHOLOGY
Histologic Features
Architectural patterns
Important to recognize
Grade is more important for prognosis; high-grade DCIS can have any architectural pattern
Majority of cases of DCIS consist of > 1 architectural type
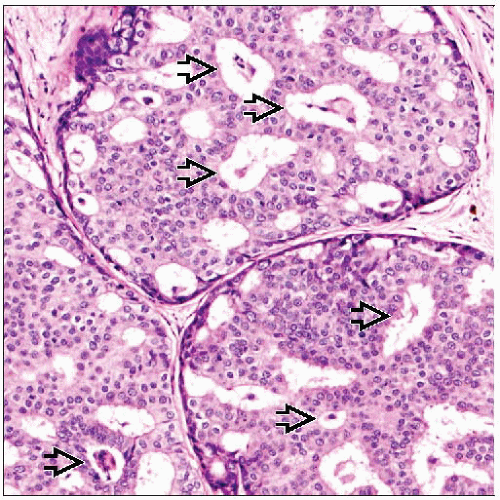
Cribriform DCIS
Cribriform lumens appear punched out and rounded in shape
In 3 dimensions, spaces are spherical
Cells should be oriented around lumen
Lumens are distributed evenly throughout involved duct
Spaces associated with hyperplasia are typically sinuous in shape and peripherally located
Papillary DCIS
Papillary fronds have a central fibrovascular core
Myoepithelial cells are present around periphery of spaces but not within papillary cores
Endothelial cells lining blood vessels can be apposed to base of tumor cells in thin fibrovascular cores
Can be difficult to distinguish endothelial cells from myoepithelial cells using IHC muscle-type markers
p63 is a better marker to confirm absence of myoepithelial cells in papillary lesions
Micropapillary DCIS
Papillae have narrow bases that expand to bulbous ends
Appearance has been compared to that of light bulb or drumstick
Papillae do not have fibrovascular cores
Surrounding duct usually lacks hyperplasia and is flat
Comedo DCIS
Central area of necrosis surrounded by rim of tumor cells
Strict definition of comedo DCIS requires both central necrosis and high nuclear grade
Calcifications are almost always present in necrotic material and are usually numerous
Associated mammographic finding: Clustered or linear and branching calcifications
Mammographic lesion is often close to actual extent of comedo DCIS
Associated circumferential stromal fibrosis, often with lymphocytic infiltrate
Some cases form a clinically palpable mass or mammographic density and can sometimes be visible as foci of necrosis in an area of firm gray-white stroma
Solid DCIS
Cells completely fill ductal spaces
Some have solid papillary pattern, as fibrovascular cores may be present within cell proliferation
Solid DCIS may show some morphologic overlap with LCIS
Clinging DCIS
Cells line spaces and do not form architectural patterns
Difficult to diagnose in isolation unless cells are of high nuclear grade
In general, more easily diagnosed architectural patterns are also present
Cytologic Features
DCIS is a clonal population, which should be reflected in morphologic appearance
In contrast, hyperplasias consist of a mixture of luminal, myoepithelial, and intermediate-type cells
Metaplasia makes recognition of DCIS very difficult
Apocrine and clear cell metaplasia impart a very uniform appearance, even in benign lesions
Architectural features, high-grade nuclei, necrosis, or associated similar-appearing invasive carcinoma may be necessary for definitive diagnosis as DCIS
Nuclear grade is important feature to classify DCIS
Same nuclear grading system used for invasive carcinoma can be used for DCIS
Often a mixture of nuclear grades; highest grade present should be reported
Mucin production can be associated with cribriform, micropapillary, papillary, or clinging DCIS
Calcifications may be present in mucin
If duct rupture, can be difficult to distinguish extravasated mucin from small foci of invasion
Tumor cells should not be present in extravasated mucin
Necrosis
Presence of necrosis is often used to classify DCIS
Comedo necrosis should involve majority of central portion of involved duct
Focal necrosis may involve only small portion of duct
Single cell necrosis can also be seen
Necrosis is always associated with comedo DCIS, but varying degrees can be seen with other types
Extent of DCIS
“Extent” refers to volume of breast tissue occupied by DCIS
Extent can vary from 0.2 cm to > 20 cm or all 4 quadrants of breast
Average extent of DCIS is 2-3 cm
Measure of extent is useful clinically to determine
Likelihood of being able to achieve breast conservation with adequate margins
Likelihood of an area of invasion being present or being missed
Minimal extent required for a diagnosis of DCIS (rather than ADH) has been proposed
0.2 cm or 2 completely involved ductal spaces have been suggested
No minimal extent required for DCIS with high-grade nuclei
Extent can only be estimated
Breast tissue is highly compressible
Shape of breast specimens changes (slumps) after excision
Specimen radiography also compresses and distorts shape (size) of excisions
Morphologic gaps in ductal involvement are reported to occur, particularly in lower grades of DCIS
DCIS is often removed in multiple specimens
Multiple methods to estimate extent
Measurement of DCIS on glass slide: Only accurate if DCIS is only present on 1 slide
Margins: If 2 opposing margins are positive or close to DCIS, extent can be estimated by using specimen size
Complete serial sequential sectioning (SSS) and mapping of sections with DCIS to give a linear measurement
Counting block method (CBM) multiplies number of blocks with DCIS by 0.4 cm (or average width of sliced tissue in cassettes, if known)
SSS correlates with CBM up to approximately 3 cm
Because CBM is related to volume as well as to linear dimension, it usually gives a larger estimate compared with SSS for very extensive DCIS
Sentinel node biopsy may be performed when DCIS is extensive, particularly as part of mastectomy
ANCILLARY TESTS
Immunohistochemistry
Myoepithelial markers
Very helpful to distinguish DCIS from invasive carcinoma and to identify microinvasion
Myoepithelial cells associated with DCIS may lose expression of some markers
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree