High-Yield Terms to Learn
Bigeminy An arrhythmia consisting of normal sinus beats coupled with ventricular extrasystoles, that is, “twinned” beats (Figure 13-4) End-diastolic fiber length The length of the ventricular fibers at the end of diastole; a determinant of the force of the following contraction Heart failure A condition in which the cardiac output is insufficient for the needs of the body. Low-output failure may be due to decreased stroke volume (systolic failure) or decreased filling (diastolic failure) PDE inhibitor Phosphodiesterase inhibitor; a drug that inhibits one or more enzymes that degrade cAMP (and other cyclic nucleotides). Examples: high concentrations of theophylline, inamrinone Premature ventricular beats An abnormal beat arising from a cell below the AV node—often from a Purkinje fiber, sometimes from a ventricular fiber Sodium pump (Na + /K + ATPase)
A transport molecule in the membranes of all vertebrate cells; responsible for the maintenance of normal low intracellular sodium and high intracellular potassium concentrations; it uses ATP to pump these ions against their concentration gradients Sodium-calcium exchanger A transport molecule in the membrane of many cells that pumps one calcium atom outward against its concentration gradient in exchange for three sodium ions moving inward down their concentration gradient Ventricular function curve The graph that relates cardiac output, stroke volume, etc, to filling pressure or end-diastolic fiber length; also known as the Frank-Starling curve Ventricular tachycardia An arrhythmia consisting entirely or largely of beats originating below the AV node
Pathophysiology
Heart failure is an extremely serious cardiac condition associated with a high mortality rate. The fundamental physiologic defect in heart failure is a decrease in cardiac output relative to the needs of the body, and the major manifestations are dyspnea and fatigue. The causes of heart failure are still not completely understood. In some cases, it can be ascribed to simple loss of functional myocardium, as in myocardial infarction. It is frequently associated with chronic hypertension, valvular disease, coronary artery disease, and a variety of cardiomyopathies. In about one third of cases of heart failure, the primary defect is a reduction of cardiac contractile force and ejection fraction that is detected during systole (systolic failure). In another third, the primary defect is stiffening or other changes of the ventricles that prevent adequate filling during diastole; ejection fraction may be normal even though stroke volume is decreased (diastolic failure). The remainder of cases can be attributed to a combination of systolic and diastolic dysfunction. The natural history of heart failure is characterized by a slow deterioration of cardiac function, punctuated by episodes of acute cardiac decompensation that are often associated with pulmonary or peripheral edema or both (congestion).
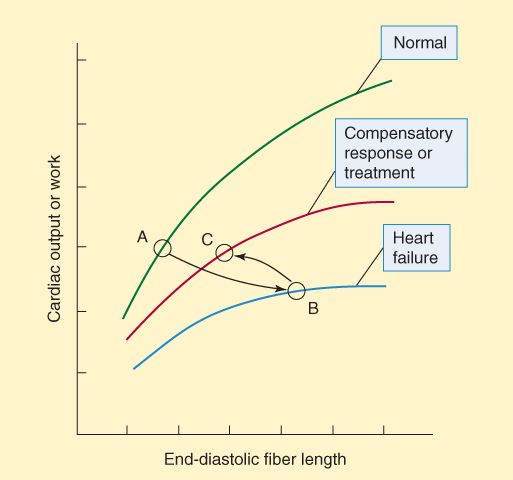
The reduction in cardiac output is best shown by the ventricular function curve (Frank-Starling curve; Figure 13-1). The changes in the ventricular function curve reflect some compensatory responses of the body and may also be used to demonstrate the response to drugs. As ventricular ejection decreases, the end-diastolic fiber length increases, as shown by the shift from point A to point B in Figure 13-1. Operation at point B is intrinsically less efficient than operation at shorter fiber lengths because of the increase in myocardial oxygen requirement associated with increased fiber stretch (see Figure 12-1).
FIGURE 13-1
Ventricular function (Frank-Starling) curves. The abscissa can be any measure of preload: fiber length, filling pressure, pulmonary capillary wedge pressure, etc. The ordinate is a measure of useful external cardiac work: stroke volume, cardiac output, etc. In heart failure, output is reduced at all fiber lengths, and the heart expands because ejection fraction is decreased or filling pressure is increased (or both). As a result, the heart moves from point A to point B. Compensatory sympathetic discharge or effective treatment allows the heart to eject more blood, and the heart moves to point C on the middle curve.
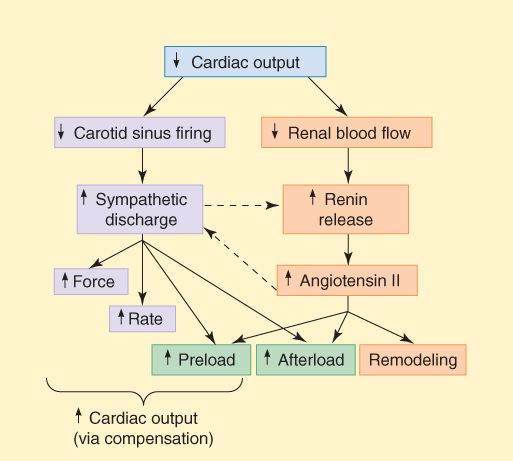
The homeostatic responses of the body to depressed cardiac output are extremely important and are mediated mainly by the sympathetic nervous system and the renin-angiotensin-aldosterone system. They are summarized in Figure 13-2. The major responses include the following: (1) Tachycardia—an early manifestation of increased sympathetic tone. (2) Increased peripheral vascular resistance—another early response, also mediated by increased sympathetic tone. (3) Retention of salt and water by the kidney—an early compensatory response, mediated by the renin-angiotensin-aldosterone system and facilitated by increased sympathetic outflow. Increased blood volume results in edema and pulmonary congestion and contributes to the increased end-diastolic fiber length. (4) Cardiomegaly (enlargement of the heart)—a slower compensatory response, mediated at least in part by sympathetic discharge and angiotensin II. Although these compensatory responses can temporarily improve cardiac output (point C in Figure 13-1), they also increase the load on the heart, and the increased load contributes to further long-term decline in cardiac function. (5) Apoptosis is a later response, and results in a reduction in the number of functioning myocytes and their replacement by connective tissue. Evidence suggests that catecholamines, angiotensin II, and aldosterone play a direct role in these changes.
FIGURE 13-2
Compensatory responses that occur in heart failure. These responses play an important role in the progression of the disease. Dashed arrows indicate interactions between the sympathetic and the renin-angiotensin systems.
Modified and reproduced, with permission, from Katzung BG, editor: Basic & Clinical Pharmacology, 11th ed. McGraw-Hill, 2009: Fig. 13-2.)
Therapeutic Strategies
Pharmacologic therapies for heart failure include the removal of retained salt and water with diuretics; reduction of afterload and salt and water retention by means of angiotensin-converting enzyme (ACE) inhibitors; reduction of excessive sympathetic stimulation by means of 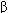 blockers; reduction of preload or afterload with vasodilators; and in systolic failure, direct augmentation of depressed cardiac contractility with positive inotropic drugs such as digitalis glycosides. Considerable evidence indicates that angiotensin antagonists, some
blockers; reduction of preload or afterload with vasodilators; and in systolic failure, direct augmentation of depressed cardiac contractility with positive inotropic drugs such as digitalis glycosides. Considerable evidence indicates that angiotensin antagonists, some 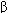 -adrenoceptor blockers, and the aldosterone antagonists spironolactone and eplerenone also have long-term beneficial effects. The use of diuretics is discussed in Chapter 15.
-adrenoceptor blockers, and the aldosterone antagonists spironolactone and eplerenone also have long-term beneficial effects. The use of diuretics is discussed in Chapter 15.
Current clinical evidence suggests that acute heart failure should be treated with a loop diuretic; if very severe, a prompt-acting positive inotropic agent such as a 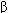 agonist or phosphodiesterase inhibitor and vasodilators should be used as required to optimize filling pressures and blood pressure. Chronic failure is best treated with diuretics (often a loop agent plus spironolactone) plus an ACE inhibitor and, if tolerated, a
agonist or phosphodiesterase inhibitor and vasodilators should be used as required to optimize filling pressures and blood pressure. Chronic failure is best treated with diuretics (often a loop agent plus spironolactone) plus an ACE inhibitor and, if tolerated, a 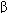 blocker. Digitalis may be helpful if systolic dysfunction is prominent. Nesiritide, a recombinant form of brain natriuretic peptide, has vasodilating and diuretic properties and has been heavily promoted for use in acute failure.
blocker. Digitalis may be helpful if systolic dysfunction is prominent. Nesiritide, a recombinant form of brain natriuretic peptide, has vasodilating and diuretic properties and has been heavily promoted for use in acute failure.
Cardiac Glycosides
Digitalis glycosides are no longer considered first-line drugs in the treatment of heart failure. However, because they are not discussed elsewhere in this book, we begin our discussion with this group.
Prototypes and Pharmacokinetics
All cardiac glycosides include a steroid nucleus and a lactone ring; most also have one or more sugar residues. The cardiac glycosides are often called “digitalis” because several come from the digitalis (foxglove) plant. Digoxin is the prototype agent and the only one commonly used in the United States. A very similar molecule, digitoxin, which also comes from the foxglove, is no longer available in the United States. Digoxin has an oral bioavailability of 60-75%, and a half-life of 36-40 h. Elimination is by renal excretion (about 60%) and hepatic metabolism (40%).
Mechanism of Action
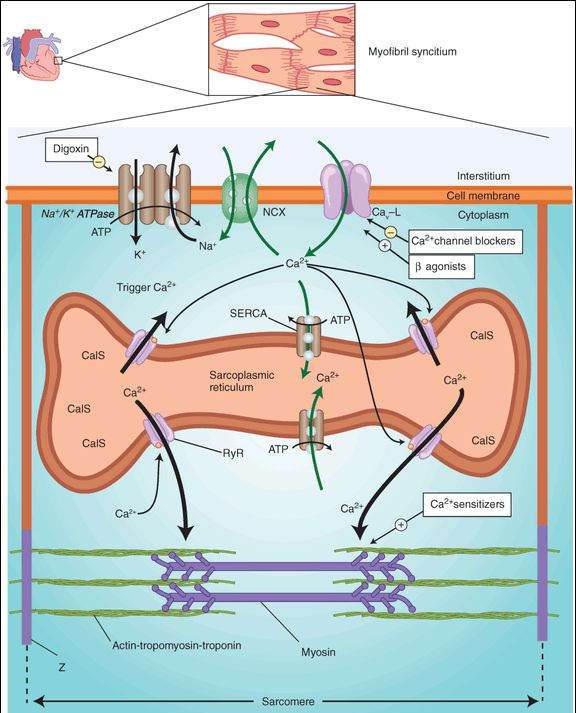
Inhibition of Na+/K+ ATPase of the cell membrane by digitalis is well documented and is considered to be the primary biochemical mechanism of action (Figure 13-3). Inhibition of Na+/K+ ATPase results in a small increase in intracellular sodium. The increased sodium alters the driving force for sodium-calcium exchange by the exchanger, NCX, so that less calcium is removed from the cell. The increased intracellular calcium is stored in the sarcoplasmic reticulum and upon release increases contractile force. Other mechanisms of action for digitalis have been proposed, but they are probably not as important as the inhibition of ATPase. The consequences of Na+/K+ ATPase inhibition are seen in both the mechanical and the electrical function of the heart. Digitalis also modifies autonomic outflow, and this action has effects on the electrical properties of the heart.
FIGURE 13-3
Schematic diagram of a cardiac sarcomere with the cellular components involved in excitation-contraction coupling and the sites of action of several drugs. Factors involved in excitation-contraction coupling include Na+/K+ ATPase; Na+/Ca2+
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree