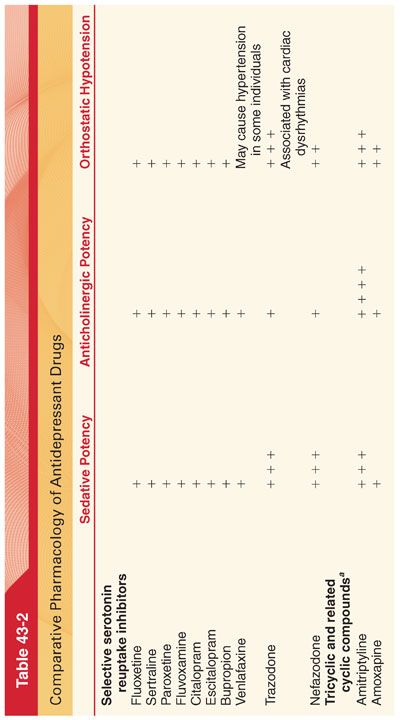
A. Selective serotonin reuptake inhibitors (SSRIs) are the most broadly prescribed class of antidepressants and are the drugs of choice for the treatment of mild to moderate depression. Compared with tricyclic antidepressants, SSRIs lack anticholinergic properties, do not cause postural hypotension or delayed conduction of cardiac impulses, and do not appear to have a major effect on the seizure threshold. Perhaps the most important advantage of SSRIs compared with tricyclic antidepressants is the relative safety of SSRIs when taken in overdose. Abrupt discontinuation of SSRIs with short elimination half-times may be associated with dizziness, paresthesias, myalgias, irritability, insomnia, and visual disturbances. Suicidal tendencies in children and adolescents may be increased in those age groups when they are treated with SSRIs.
1. Fluoxetine is commonly administered once daily in the morning to decrease the risk of insomnia. Because fluoxetine has a prolonged elimination half-time (1 to 3 days for acute administration and 4 to 6 days for chronic administration), the drug can be taken every other day.
a. Side Effects. The most common side effects of fluoxetine are nausea, anorexia, insomnia, sexual dysfunction, agitation, and neuromuscular restlessness, which may mimic akathisia. Like tricyclic antidepressants, fluoxetine may be an effective analgesic for treatment of chronic pain as may be associated with rheumatoid arthritis. Fluoxetine does not cause hypotension, and changes in conduction of cardiac impulses seem infrequent. Because of its long elimination half-time, fluoxetine should be discontinued for about 5 weeks before initiating treatment with an MAO inhibitor. The long elimination half-time of fluoxetine appears to prevent withdrawal symptoms induced by abrupt discontinuance of the drug. An overdose with fluoxetine alone is not associated with the risk of cardiovascular and central nervous system (CNS) toxicity.
b. Drug Interactions. Among the SSRIs, fluoxetine is the most potent inhibitor of certain hepatic cytochrome P450 enzymes. As a result, this drug may increase the plasma concentrations of drugs that depend on hepatic metabolism for clearance (addition of fluoxetine to treatment with a tricyclic antidepressant drug may result in a two- to fivefold increase in the plasma concentration of the tricyclic drug). MAO inhibitors combined with fluoxetine may cause the development of a serotonin syndrome characterized by anxiety, restlessness, chills, ataxia, and insomnia.
2. Sertraline has a spectrum of efficacy similar to fluoxetine. This drug has a shorter elimination half-time (25 hours) than fluoxetine and is a less potent inhibitor of hepatic microsomal enzymes. The recommended washout period before starting an MAO inhibitor is 14 days.
3. Paroxetine has an efficacy similar to that of fluoxetine. This drug has a relatively short elimination half-time (24 hours), and there are no active metabolites. Side effects resemble those of other SSRIs with the exception of a possibly increased incidence of sedation. Paroxetine produces less inhibition of hepatic cytoplasmic P450 enzymes than is fluoxetine. Enhancement of the anticoagulant effect of warfarin reflects competition for common protein-binding sites. The recommended washout period before starting an MAO inhibitor is 14 days.
4. Citalopram/Escitalopram (S Isomer of Citalopram). Citalopram causes dose-dependent QT interval prolongation, which can place patients at risk for torsades de pointes. Escitalopram may also prolong the QT interval but possibly to a lesser degree.
5. Fluvoxamine is effective in the management of obsessive-compulsive disorders. Although it produces less inhibition of hepatic cytoplasmic P450 enzymes than the other SSRIs, fluvoxamine may still cause clinically significant drug interactions.
6. Bupropion is effective in the treatment of major depression, producing improvement in 2 to 4 weeks. In addition, bupropion is effective for smoking cessation. Unlike the SSRIs, bupropion is not associated with significant drug interactions and is not commonly associated with sexual dysfunction.
7. Venlafaxine is perceived to have a profile of efficacy similar to that of the tricyclic antidepressants but does not produce anticholinergic effects or postural hypotension.
8. Duloxetine. Indications for its use include major depression, fibromyalgia, and diabetic neuropathy. Duloxetine should be avoided in patients with severe renal dysfunction and chronic liver disease. The potential for the development of serotonin syndrome is present when this drug is used in conjunction with another serotonergic drug.
9. Trazodone is effective in the management of depression but its greatest efficacy may be treatment of insomnia induced by SSRIs or bupropion.
10. Nefazodone is chemically related to trazodone but with fewer α1-adrenergic blocking properties. The risk of sedation and priapism may be less than in patients treated with trazodone. Orthostatic hypotension may occur.
B. Management of Anesthesia
1. SSRIs may have antiplatelet activity and increase the risk of bleeding particularly in the setting of antiplatelet medication use.
2. The risks of discontinuing an SSRI may take 2 to 3 weeks for full washout and reinitiation may require 2 to 4 weeks for reestablishment of clinical antidepressant effect. Furthermore, discontinuation of a patient’s SSRI may expose them to the risks of a major depressive episode.
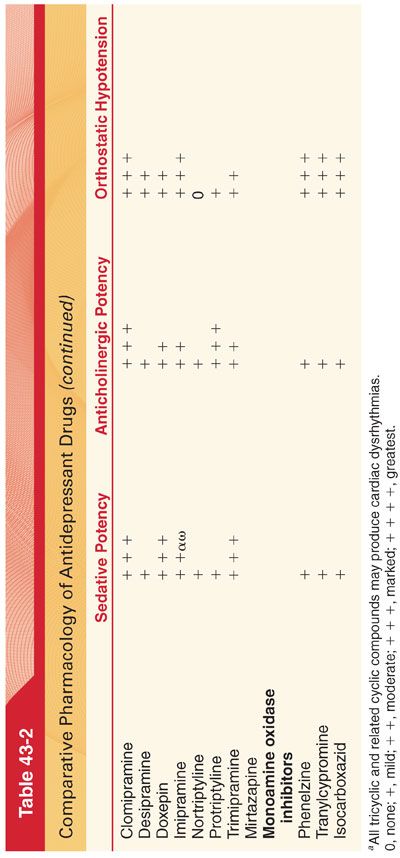
C. Tricyclic and Related Antidepressants. Before the availability of SSRIs, tricyclic antidepressants and related cyclic antidepressants were the most commonly used drugs to treat depression (see Table 43-2). Although tricyclic antidepressants are highly effective, they have been supplanted as first-line drugs in many clinical situations because of their unfavorable side effect profile (largely resulting from their anticholinergic, antiadrenergic, and antihistaminic properties). Tricyclic antidepressants also have a narrow therapeutic index and are potentially lethal in overdose.
1. Chronic Pain Syndromes. The tricyclic antidepressants (especially amitriptyline and imipramine), in doses lower than those used to treat depression, may be useful in the treatment of chronic neuropathic pain and other chronic pain syndromes including fibromyalgia. The efficacy of tricyclic antidepressants on chronic pain syndromes may be limited by a narrow therapeutic index and intolerability of side effects.
2. Structure–Activity Relationships. The structure of tricyclic antidepressants resembles that of local anesthetics and phenothiazines.
a. Imipramine, which is the prototype of the tricyclic antidepressants, differs from phenothiazine only in the replacement of the sulfur atom with an ethylene linkage to produce a seven-membered central ring.
b. Desipramine is the principal metabolite of imipramine, and nortriptyline is the demethylated metabolite of amitriptyline.
3. Mechanism of Action. Tricyclic antidepressants act at several transporters and receptors, but their antidepressant effect is likely produced by blocking the reuptake (uptake) of serotonin and/or norepinephrine at presynaptic terminals, thereby increasing the availability of these neurotransmitters.
4. Pharmacokinetics. Tricyclic antidepressants are efficiently absorbed from the gastrointestinal tract after oral administration, reflecting high lipid solubility. The long elimination half-time (17 to 30 hours) and wide range of therapeutic plasma concentrations make once-daily dosing intervals effective.
5. Metabolism. Tricyclic antidepressants are oxidized by microsomal enzymes in the liver with subsequent conjugation with glucuronic acid. Imipramine is metabolized to the active compound desipramine.
6. Side Effects (see Table 43-2)
a. Anticholinergic effects of tricyclic antidepressants are prominent, especially at high doses. Amitriptyline causes the highest incidence of anticholinergic effects (dry mouth, blurred vision, tachycardia, urinary retention, slowed gastric emptying, ileus), whereas desipramine produces the fewest such effects.
b. Cardiovascular Effects. Orthostatic hypotension and modest increases in heart rate are the most common cardiovascular side effects of tricyclic antidepressants (orthostatic hypotension may be particularly hazardous in elderly patients, who are at increased risk of fractures when they fall). The risk of hypotension during general anesthesia in patients treated with tricyclic antidepressants is low. Previous suggestions that tricyclic antidepressants increase the risks of cardiac dysrhythmias and sudden death have not been substantiated in the absence of drug overdose. Atropine is a useful treatment when tricyclic antidepressants dangerously slow atrioventricular or intraventricular conduction of cardiac impulses. Direct cardiac depressant effects may reflect quinidine-like actions of tricyclic antidepressants on the heart.
c. Central Nervous System Effects. Sedation associated with tricyclic antidepressant therapy may be desirable for management of depressed patients with insomnia. Tricyclic antidepressants lower the seizure threshold, raising the question of the advisability of administering these drugs to patients with seizure disorders. Treatment with tricyclic antidepressants may enhance the CNS-stimulating effects of enflurane. Because of their cardiac toxicity, tendency to cause seizures, and depressant properties on the CNS, the tricyclic antidepressants may be fatal if taken in an overdose. The combination of a tricyclic antidepressant and an MAO inhibitor may result in CNS toxicity manifesting as hyperthermia, seizures, and coma.
7. Drug Interactions
a. Sympathomimetics. For patients recently started on tricyclic antidepressants, exaggerated pressor responses should be anticipated whether or not direct-acting or indirect-acting sympathomimetics are administered, whereas in individuals chronically treated with tricyclic antidepressants (>6 weeks), administration of either a direct-acting or indirect-acting sympathomimetic is acceptable. Induction of anesthesia may be associated with an increased incidence of cardiac dysrhythmias in patients treated with tricyclic antidepressants. The dose of exogenous epinephrine necessary to produce cardiac dysrhythmias during anesthesia with a volatile anesthetic is decreased by tricyclic antidepressants.
b. Anticholinergics. Because the anticholinergic side effects of drugs may be additive, the use of centrally active anticholinergic drugs for preoperative medication of patients treated with tricyclic antidepressants could increase the likelihood of postoperative delirium and confusion (central anticholinergic syndrome). Glycopyrrolate would theoretically be less likely to evoke this type of drug interaction in patients being treated with tricyclic antidepressants.
c. Antihypertensives. Rebound hypertension after abrupt discontinuation of clonidine may be accentuated and prolonged by concomitant tricyclic antidepressant therapy.
d. Opioids. In animals, tricyclic antidepressants augment the analgesic and ventilatory depressant effects of opioids.
8. Tolerance to anticholinergic effects (dry mouth, blurred vision, tachycardia) and orthostatic hypotension develops during chronic therapy with tricyclic antidepressants.
9. Overdose
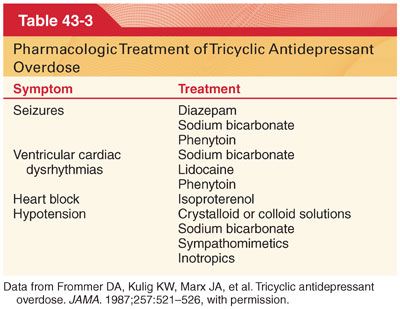
a. Tricyclic antidepressant overdose is life-threatening, as the progression from an alert state to unresponsiveness may be rapid. Intractable myocardial depression or ventricular cardiac dysrhythmias are the most frequent terminal events.
b. Presenting features of tricyclic antidepressant overdose include agitation and seizures followed by coma, depression of ventilation, hypotension, hypothermia, and striking evidence of anticholinergic effects including mydriasis, flushed dry skin, urinary retention, and tachycardia. The QRS complex on the electrocardiogram (ECG) may be prolonged to greater than 100 milliseconds.
c. Treatment of a life-threatening overdose of a tricyclic antidepressant is directed toward management of CNS and cardiac toxicity (Table 43-3).
D. Monoamine Oxidase Inhibitors. MAO inhibitors constitute a heterogenous group of drugs that block the enzyme that metabolizes biogenic amines, increasing the availability of these neurotransmitters in the CNS and peripheral autonomic nervous system. MAO inhibitors are used less commonly because their administration is complicated by side effects (hypotension), lethality in overdose, and lack of simplicity in dosing. Patients treated with MAO inhibitors must follow a specific tyramine-free diet because of the potential for pharmacodynamic interactions with tyramine that can result in systemic hypertension (Table 43-4).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


