19
Drug Therapy of Thromboembolic Disorders
This chapter will be most useful after having a basic understanding of the material in Chapter 30, Blood Coagulation and Anticoagulant, Fibrinolytic, and Antiplatelet Drugs in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. In particular, the reader is directed to the following table and to animations available in the online version of Goodman & Gilman’s The Pharmacological Basis of Therapeutics:
• Table 30-3 Absolute and Relative Contraindications to Fibrinolytic Therapy.
• Several animations in the online version of Goodman & Gilman’s The Pharmacological Basis of Therapeutics illustrate the mechanism of action of many anticoagulant, fibrinolytic, and antiplatelet drugs.
LEARNING OBJECTIVES
 Know the classes of drugs that inhibit platelet function, their mechanisms of action, and their role in the prevention and treatment of acute coronary syndromes, strokes, and transient ischemic attacks.
Know the classes of drugs that inhibit platelet function, their mechanisms of action, and their role in the prevention and treatment of acute coronary syndromes, strokes, and transient ischemic attacks.
 Know the classes of drugs used as anticoagulants, their mechanisms of action, their indications in preventing and treating venous thromboembolism, pulmonary embolism, and strokes.
Know the classes of drugs used as anticoagulants, their mechanisms of action, their indications in preventing and treating venous thromboembolism, pulmonary embolism, and strokes.
 Know how anticoagulant drug therapy is monitored.
Know how anticoagulant drug therapy is monitored.
 Know the mechanism of action of fibrinolytic agents, and their indications in treating ischemic stroke and myocardial infarction.
Know the mechanism of action of fibrinolytic agents, and their indications in treating ischemic stroke and myocardial infarction.
 Know the major toxicities and contraindications of each class of drug.
Know the major toxicities and contraindications of each class of drug.
DRUGS INCLUDED IN THIS CHAPTER
• Abciximab (REOPRO)
• Apixaban (ELIQUIS)
• Argatroban (ARGATROBAN)
• Aspirin (acetylsalicylic acid, ASA)
• Bivalirudin (ANGIOMAX)
• Clopidogrel (PLAVIX)
• Dabigatran (PRADAXA)
• Drotrecogin alfa (XIGRIS)
• Eptifibatide (INTEGRILIN)
• Fondaparinux (ARIXTRA)
• Heparin
• Low-Molecular-Weight Heparins (LMWHs; enoxaparin [LOVENOX], dalteparin [FRAGMIN], tinzaparin [INNOHEP], ardeparin [NORMIFLO], nadroparin [FRAXIPARIN])
• Prasugrel (EFFIENT)
• Rivaroxaban (XARELTO)
• Ticlopidine (TICLID)
• Tirofiban (AGGRASTAT)
• Tissue plasminogen activator (t-PA; alteplase [ACTIVASE], reteplase [RETEVASE], tenecteplase [TNKASE])
• Warfarin (COUMADIN)
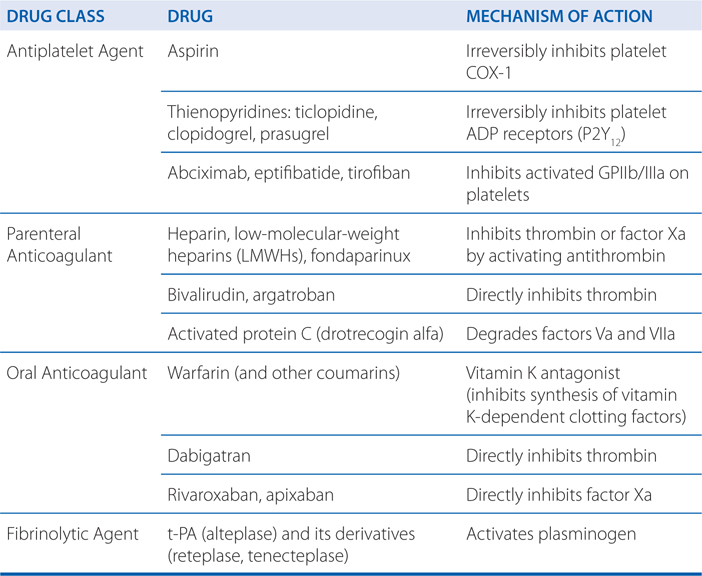
MECHANISMS OF ACTION OF DRUGS COMMMONLY USED TO PREVENT OR TREAT THROMBOEMBOLISM
COMMON INDICATIONS FOR THE USE OF ANTIPLATELET DRUGS
• Prevention of platelet thrombi after coronary angioplasty
• Secondary prevention of myocardial infarction, transient ischemic attacks (TIAs), and stroke
• Primary prevention of myocardial infarction, TIAs, and stroke in high-risk patients
A 62-year-old man with a history of angina calls 911 complaining of crushing chest pain. The 911 operator sends an ambulance and instructs the patient to chew an aspirin while waiting for the ambulance to arrive.
a. Why did the 911 operator have the patient take an aspirin?
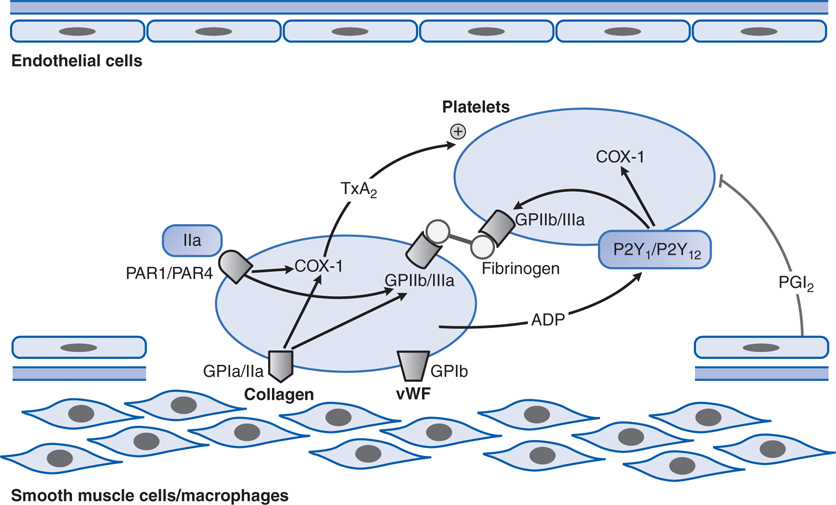
The patient’s history of angina suggests he has coronary artery disease and an elevated risk of an MI, and his symptoms of crushing chest pain indicate he may be having a myocardial infarction. In a patient with long-standing coronary artery disease, coronary vessel wall damage resulting from a ruptured atherosclerotic plaque can expose collagen, vWF, and other subendothelial prothrombotic substances to the blood (see Figures 19-1 and 19-2); these subendothelial substances will bind and activate circulating platelets. The activated platelets will release a number of mediators, including thromboxane A2 (TXA2), which will activate other platelets in the blood vessel and stimulate the platelets to aggregate at the site of the vessel wall damage. Aspirin can inhibit platelet production of TXA2 by irreversibly inhibiting platelet COX-1. By chewing an aspirin to increase absorption in the GI tract, the patient will rapidly inhibit platelet activation and aggregation, and slow the development of a platelet thrombus that may lead to the occlusion of a major coronary vessel.
FIGURE 19-1 Platelet adhesion and aggregation. GPIa/IIa and GPIb are platelet receptors that bind to collagen and von Willebrand factor (vWF), causing platelets to adhere to the subendothelium of a damaged blood vessel. PAR1 and PAR4 are protease-activated receptors that respond to thrombin (IIa); P2Y1 and P2Y12 are receptors for ADP; when stimulated by agonists, these receptors activate the fibrinogen-binding protein GPIIb/IIIa and cyclooxygenase-1 (COX-1) to promote platelet aggregation and secretion. Thromboxane A2 (TxA2) is the major product of COX-1 involved in platelet activation. Prostaglandin I2 (prostacyclin, PGI2), synthesized by endothelial cells, inhibits platelet activation.
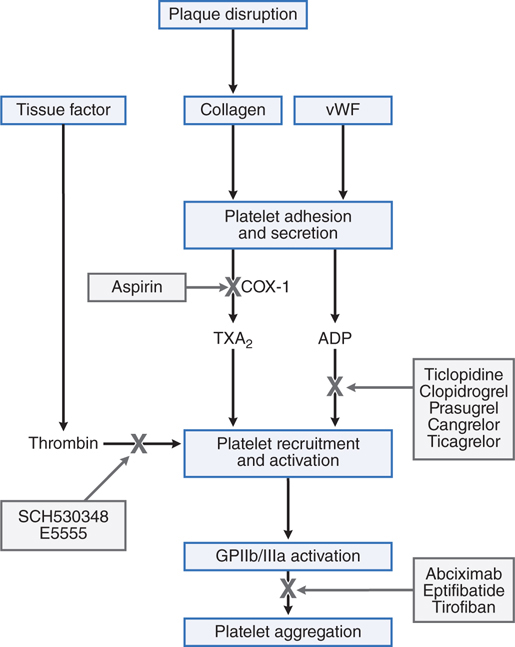
FIGURE 19-2 Sites of action of antiplatelet drugs. Aspirin inhibits thromboxane A2 (TxA2) synthesis by irreversibly acetylating cyclooxygenase-1 (COX-1). Reduced TxA2 release attenuates platelet activation and recruitment to the site of vascular injury. Ticlopidine, clopidogrel, and prasugrel irreversibly block P2Y12, a key ADP receptor on the platelet surface; cangrelor and ticagrelor are reversible inhibitors of P2Y12. Abciximab, eptifibatide, and tirofiban inhibit the final common pathway of platelet aggregation by blocking fibrinogen and von Willebrand factor (vWF) from binding to activated glycoprotein (GP) IIb/IIIa. SCH530348 (now known as vorapaxar [ZANTIVITY]) and E5555 (also known as atopaxar) inhibit thrombin-mediated platelet activation by targeting protease-activated receptor-1 (PAR-1), the major thrombin receptor on platelets.
b. When the patient arrives at the hospital, he is diagnosed as having a myocardial infarction and undergoes percutaneous angioplasty to clear the clot in his coronary artery. He also receives a stent to keep the vessel patent. What drugs will this patient likely receive while in the hospital to prevent recurrence of a thrombus in the stented coronary vessel?
The patient will receive aggressive antiplatelet and anticoagulant therapy in the hospital to reduce the likelihood of platelet and fibrin clots in the stented coronary vessel. To prevent platelet thrombi, he would continue to receive aspirin as well as more efficacious antiplatelet inhibitors, including an ADP receptor antagonist (ie, clopidogrel or prasugrel) and possibly a GPIIb/IIIa inhibitor (ie, abciximab, eptifibatide, or tirofiban). To inhibit the formation of fibrin clots, the patient would receive heparin or an LMWH. He would also be started on an oral anticoagulant (ie, warfarin) before discharge but kept on the heparinoid until his INR (see Side Bar LABORATORY MONITORING OF ANTICOAGULANT THERAPY) is within the therapeutic range.
BIOACTIVATION OF THIENOPYRIDINES (TICLOPIDINE, CLOPIDOGREL, AND PRASUGREL)
• All thienopyridines are prodrugs that require bioactivation by CYPs and/or esterases
• There is wide interindividual variability in antiplatelet effects of clopidogrel because the prodrug is primarily activated by CYP2C19 which has a loss-of-function polymorphism (CYP2C19*2) and several reduced-function polymorphisms (CYP2C19*3, *4, *5)
• Prasugrel has rapid and nearly complete absorption and activation compared with only 15% activation of absorbed clopidogrel
• Prasugrel is more efficacious and predictable than clopidogrel but is also associated with higher rates of fatal and life-threatening bleeding
COMMON INDICATIONS FOR THE USE OF ANTICOAGULANTS
• Treatment of venous thrombosis and pulmonary embolism
• Prevention of thromboembolism in patients with unstable angina or acute myocardial infarction
• Prevention of thromboembolism in patients with atrial fibrillation
• Prevention of postoperative deep vein thrombosis in patients undergoing abdominothoracic surgery, and knee and hip replacements
• Cardiopulmonary bypass surgery to prevent clotting of the oxygenator
c. What drugs is this patient likely to receive after being discharged from the hospital?
To prevent reocclusion of the stented coronary artery, he will need to take low-dose aspirin, an ADP receptor antagonist (clopidogrel or prasugrel), and warfarin. He will also be started on a β adrenergic antagonist to reduce his risk of heart failure and ventricular arrhythmias.
A 78-year-old man receives a total knee replacement. He lives by himself about an hour’s drive from the nearest medical facility. Upon discharge, he is given a prescription for lovenox.
a. What is the rationale for the use of this agent?
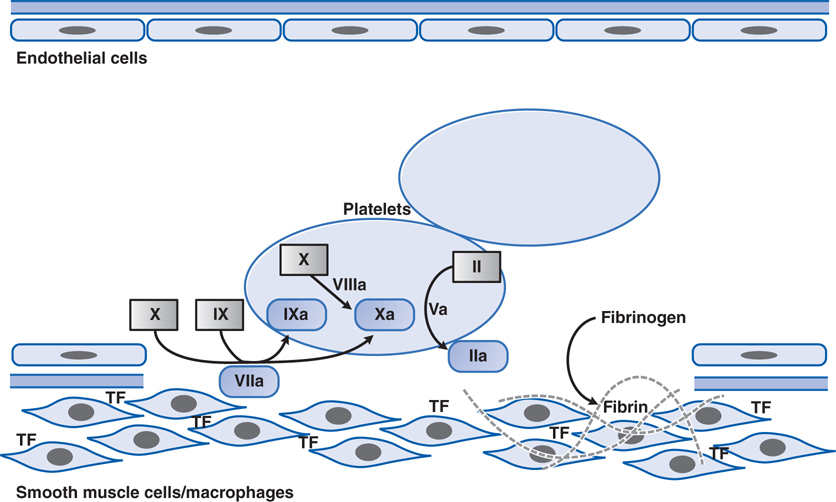
Patients having major orthopedic surgeries such as total knee replacement or hip replacement are at risk of fibrin clot formation because of the surgery which activates the clotting cascade (see Figure 19-3) and subsequent immobilization which promotes blood stasis. To minimize the risk of fibrin clot formation that could lead to pulmonary embolism or stroke, such patients receive an LMWH such as lovenox. Because this agent has very predictable pharmacokinetics, it does not require laboratory monitoring, and it can be self-administered by subcutaneous injection, and is thus well suited for this patient who lives a considerable distance from the nearest medical facility.
FIGURE 19-3 Major reactions of blood coagulation. Shown are interactions among proteins of the “extrinsic” (tissue factor and factor VII), “intrinsic” (factors IX and VIII), and “common” (factors X, V, and II) coagulation pathways that are important in vivo. Boxes enclose the coagulation factor zymogens (indicated by Roman numerals); the rounded boxes represent the active proteases. TF, tissue factor. Activated coagulation factors are followed by the letter “a”: II, prothrombin; IIa, thrombin.
b. What is the mechanism of action of lovenox?
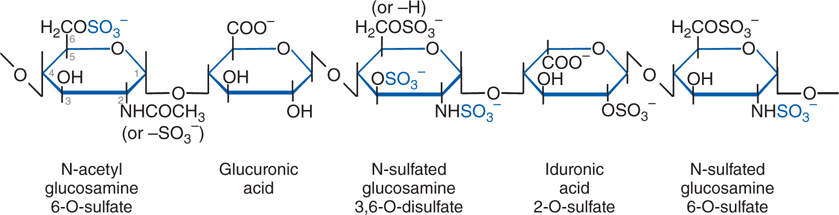
Lovenox is one of several LMWH agents and has the same mechanism of action as heparin and other heparinoids such as fondaparinux. Heparin and all of the heparinoids contain a pentasaccharide structure (see Figure 19-4) that binds and activates antithrombin, an endogenous anticoagulant (see Figure 30-5 in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition). Once activated, antithrombin inhibits the clotting factors thrombin (factor IIa) and factor Xa (see Figure 19-3). Inhibition of thrombin blocks the conversion of fibrinogen to insoluble fibrin, whereas inhibition of factor Xa inhibits conversion of the inactive prothrombin (factor II) zymogen to active thrombin (factor IIa). Because of the smaller size of the LMWHs compared to standard heparin, the LMWH-antithrombin complexes are more specific inhibitors of factor Xa than thrombin (see Figure 30-5 in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition).
FIGURE 19-4 The antithrombin-binding pentasaccharide structure of heparin. Sulfate groups required for binding to antithrombin are indicated in blue.
c. What are the important drug toxicities that this patient should be cautioned to watch for?
As with all anticoagulants, bleeding is the most important and common toxicity. Heparin-induced thrombocytopenia (HIT) is also possible with the LMWHs, but this adverse effect is much less common than with standard heparin.
A 32-year-old woman in good health is discovered to have atrial fibrillation during a routine checkup. She does not recall having any symptoms of arrhythmia such as dizziness or fainting, although she is not able to exercise for as long as she could a few years ago. Her cardiologist prescribes warfarin.
a. What is the rationale for using warfarin in this patient?
Patients with atrial fibrillation are at high risk of developing fibrin clots that can lead to transient ischemic attacks (TIAs), strokes, and pulmonary embolism. Because the atria do not contract properly in patients with atrial fibrillation, blood can pool in the atria increasing the chance a fibrin thrombus will form. Warfarin is an oral anticoagulant that can reduce the risk of fibrin clot formation.
b. What is the mechanism of action of warfarin?
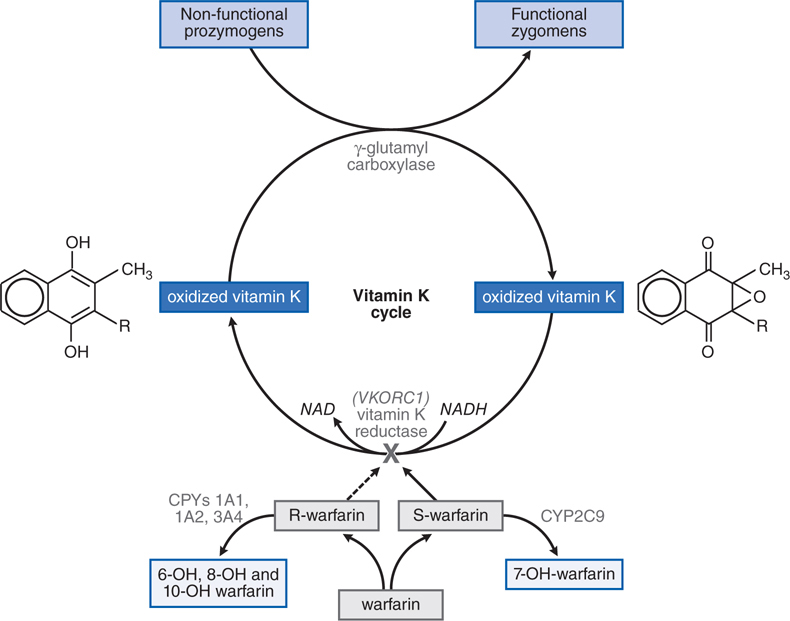
Warfarin is classified as a vitamin K antagonist. It blocks the enzyme that converts the oxidized form of vitamin K to the reduced form (see Figure 19-5). The reduced form of vitamin K is a cofactor required in the post-translational modification of clotting factors II, VII, IX, and X. This post-translational modification, γ-carboxylation of specific glutamic acids, is required for biological activity of these clotting factors. Thus, warfarin blocks the final step in the synthesis of these key coagulation factors. Therapeutic doses of warfarin reduce by 30 to 50% the total amount of each vitamin K-dependent clotting factor, and reduce the level of γ-carboxylation (and biological activity) by 10 to 40%.
FIGURE 19-5 The vitamin K cycle and mechanism of action of warfarin. In the racemic mixture of S– and R-enantiomers, S-warfarin is more active. By blocking vitamin K epoxide reductase encoded by the VKORC1 gene, warfarin inhibits the conversion of oxidized vitamin K epoxide into its reduced form, vitamin K hydroquinone. This inhibits vitamin K-dependent γ-carboxylation of factors II, VII, IX, and X because reduced vitamin K serves as a cofactor for a γ-glutamyl carboxylase that catalyzes the γ-carboxylation process, thereby converting prozymogens to zymogens capable of binding Ca2+ and interacting with anionic phospholipid surfaces. S-warfarin is metabolized by CYP2C9. Common genetic polymorphisms in this enzyme can influence warfarin metabolism. Polymorphisms in the C1 subunit of vitamin K reductase (VKORC1) also can affect the susceptibility of the enzyme to warfarin-induced inhibition, thereby influencing warfarin dosage requirements.
c. What kinds of laboratory monitoring are required for patients receiving warfarin and why?
Because many factors can alter the anticoagulant activity of warfarin (see Side Bar FACTORS LEADING TO ALTERED ANTICOAGULANT ACTIVITY OF WARFARIN), it is necessary to regularly monitor anticoagulant status using the laboratory test called the prothrombin time (PT) assay. The PT is normalized based on the activity of one of the assay reagents to give a value known as the INR (international normalized ratio). Dose adjustments of warfarin are used to maintain a patient’s INR within a narrow range of values. For most indications, the target INR is 2 to 3, but a higher target INR (2.5-3.5) is indicated in patients with high-risk mechanical heart valves. INR values that are too high indicate the patient is receiving too much warfarin and is at risk of major bleeding events. INR values that are below the desired range indicate the warfarin dosing is too low and the patient is at greater risk of stroke and pulmonary emboli. Patients who are initiated on warfarin therapy require laboratory monitoring every few days, but once a stable therapeutic INR is achieved, the frequency of monitoring can be reduced to once every few weeks.
d. What are the risks and toxicities associated with warfarin therapy?
As with all anticoagulant agents, the most common risk is bleeding. The risk of major bleeding is less than 3% in patients who are maintained within a therapeutic target INR of 2 to 3. The risk of intracranial bleeding increases markedly with INR more than 4. In patients whose INR is more than or equal to 5, vitamin K1 administration may be required to restore clotting function. Warfarin administration during pregnancy causes birth defects and abortion; heparin, LMWHs, or fondaparinux should be used instead of warfarin in pregnant women. Less common toxicities of warfarin include skin necrosis, purple toe syndrome, and other toxicities that are described in Chapter 30 of Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition.
e. What counseling should this patient receive?
Because this patient is of child-bearing age, she needs to be warned that warfarin causes birth defects and abortion. She should be changed to a heparinoid if there is a possibility she is pregnant or will become pregnant. All patients receiving warfarin should be educated to report any changes in medications (including nonprescription drugs) or food supplements because of the large number of drug–drug and food–drug interactions possible with warfarin that alter the pharmacokinetics and efficacy of warfarin’s anticoagulant properties (see Side Bar FACTORS LEADING TO ALTERED ANTICOAGULANT ACTIVITY OF WARFARIN). Dietary changes can also affect warfarin efficacy by altering the intake of vitamin K. For instance, changing to a diet that is rich in green leafy vegetables (which are high in vitamin K) can lower the patient’s INR. The patient should report any change in medications, dietary supplements, or significant change in diet so that more frequent INR testing can be done and the results used to adjust warfarin dosing.
f. Are there other oral anticoagulants that this patient might be prescribed instead of warfarin?
Because this patient has nonvalvular atrial fibrillation, alternative oral anticoagulants that could be considered include the oral direct thrombin inhibitor dabigatran etexilate, or one of the oral direct factor Xa inhibitors, rivaroxaban or apixaban. An advantage of these agents over warfarin is that they do not require routine laboratory monitoring of anticoagulant status because they have predictable pharmacokinetics and few drug–drug or food–drug interactions.
While shopping in the grocery store, a 61-year-old man develops symptoms of weakness on one side of his body and his wife notices that he has trouble speaking. Suspecting that he is having a stroke, they go to the nearest hospital which is a 20-minute drive from the store. At the hospital it is determined that the patient has had an ischemic stroke and alteplase is administered.
a. What is the rationale for administering alteplase to this patient?
Alteplase is a fibrinolytic (“clot-buster”) agent that breaks down fibrin clots. In this patient, who has an ischemic stroke, alteplase lyses the fibrin clots that are occluding blood vessels in the patient’s brain, thus restoring blood flow to the ischemic regions and preventing irreversible damage.
b. What is the mechanism of action of alteplase?
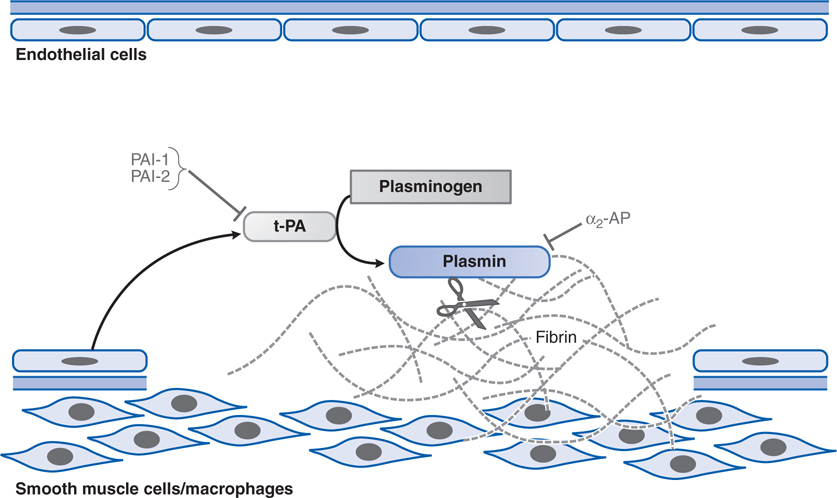
Alteplase is the recombinant form of tissue plasminogen activator (t-PA; see Figure 19-6). Alteplase and its genetically engineered derivatives, reteplase and tenecteplase, are serine proteases that cleave plasminogen to plasmin, thus activating plasmin’s proteolytic activity (see Figure 19-6). Plasmin is the endogenous protease that is responsible for digesting fibrin clots (see Figure 30-3 in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition).
FIGURE 19-6 Fibrinolysis. Endothelial cells secrete tissue plasminogen activator (t-PA) at sites of injury. t-PA binds to fibrin and converts plasminogen to plasmin, which digests fibrin. Plasminogen activator inhibitors-1 and -2 (PAI-1, PAI-2) inactivate t-PA; α2-antiplasmin (α2-AP) inactivates plasmin.
c. What must be considered before using alteplase to treat a patient with symptoms of stroke?
During the initial workup of this patient, it must be determined that this patient is having an acute ischemic stroke rather than a hemorrhagic stroke since the symptoms are similar. Administering a fibrinolytic agent to a patient with a hemorrhagic stroke would be catastrophic. It is also important to determine the time the patient first experienced symptoms because fibrinolytic therapy is maximally effective within the first 3 hours following the development of symptoms. Because of the risk of major bleeding that can occur with fibrinolysis of physiological clots, there are a number of contraindications to fibrinolytic therapy (see Table 30-3 in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition).
FACTORS LEADING TO ALTERED ANTICOAGULANT ACTIVITY OF WARFARIN
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree