Fig. 33.1
Structure of a documentation system
The purpose of the various documents is as follows:
Quality manual (see Sect. 35.6.1): the quality manual describes the quality system of the pharmacy. It sets out the structure of the organisation, the responsibilities, the policy and the facilities of the pharmacy, such as rooms and equipment. It contains an overview of all procedures in use. Some procedures may form a part of the quality manual themselves.
Procedures (see Sect. 33.3): procedures are documents that describe all actions, controls, and other measures related to a particular activity. Procedures have a general structure. Often the term ‘standard operating procedures’ (SOP) is used, although instructions or work instructions are also commonly used terms. Instructions often provide more detailed descriptions of specific actions. In this chapter, the term Standard Operating Procedures (SOPs) is preferred.
Records: a record is a paper or electronic copy of a piece of information or series of data, used by the operator to report on the work carried out.
Logbooks (see Sect. 33.7): a logbook describes chronologically the history of equipment or premises, such as operation during use, maintenance, update, renewal and repair.
File: collection of information about a specific device or a specific product (see Sect. 33.8), including technical descriptions, supporting literature, research etcetera.
33.2.3 Documentation of the Preparation
Regarding pharmacy preparations, three types of documents can be distinguished:
Preparation instructions (see Sect. 33.4), describing the formulation, the method of preparation, the batch size, the packaging and labelling of a specific pharmacy preparation. During preparation, all the actions taken are reported in a preparation record. Preparation instructions for stock preparations and extemporaneous preparations will be distinctive in format, and there will also be differences between standard preparations and non-standard preparations.
Operating instructions, describing the operation, maintenance and cleaning of a particular device or apparatus. Reporting occurs in a logbook (see Sect. 33.7), in which the user records when the equipment has been used and cleaned.
Analytical instructions (see Sect. 33.6), concern the examination of a finished product, raw material or packaging. The results of analytical testing are presented in an analytical report or certificate.
When several documents related to the same product are bundled, it is called a product file. The product file is basically a collection of information, instructions, records, investigation results etcetera on a specific product. The product file is further discussed in Sect. 33.8.
33.3 Standard Operating Procedures (SOPs)
Written SOPs are essential documents to ensure a consistent process is followed and provide detailed guidelines for various activities. They serve as guidance documents and contain generic information. SOPs can cover all parts of the quality system, including the establishment and maintenance thereof. Examples of required SOPs include:
Drafting, authorisation and implementation of SOPs
Training, assessment and qualification of personnel
Cleaning and maintenance of premises and equipment
Preparation methods
Release of preparations
Handling of complaints and deviations
Change management
Sometimes certain actions need to be described in more detail, for example when describing a specific process in a step by step manner. This kind of document is generally called a Work Instruction (WI). Examples are:
Calibration of balances;
Preparation of specific dosage forms;
Sampling for pharmaceutical analysis;
Microbiological monitoring of preparation areas.
Thus, SOPs and WIs comprise general actions and operations that apply to groups of products or equipment. The preparation of a specific medicine will be described in a Preparation Instruction (PI) (see Sect. 33.4), while maintenance of equipment is laid down in Operating Instructions. SOPs are not suitable for the recording of control data. When the activities described in SOPs or instructions produce results or other data, for example results of audits and investigations or data from calibration of equipment, these results are collected in an audit report, a record or a logbook, which is specific to the equipment being examined Sect. 33.7.
An example of a SOP is shown in Fig. 33.2. The figure gives an impression of the possible lay-out of a SOP, but other choices may be made depending on local preferences or needs. Items that are almost always required in a SOP are mentioned underneath. The numbers correspond to the sections of Fig. 33.2.
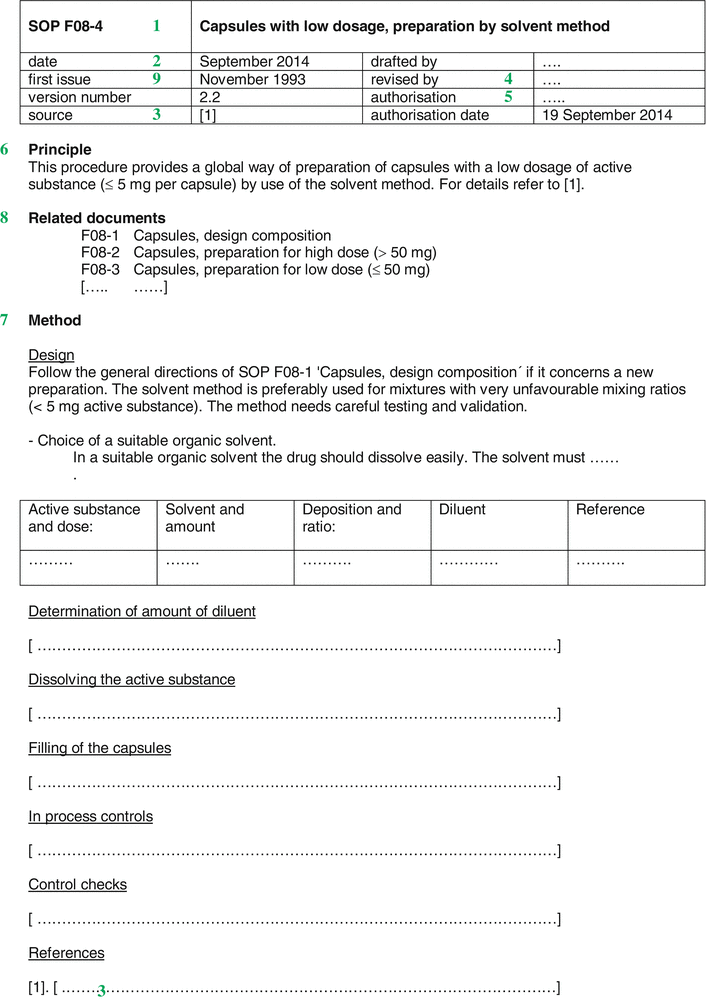

Fig. 33.2
Example of a Standard Operation Procedure (SOP)
Title (  ) )A SOP bears a unique title that gives a brief description of the subject. A unique number may be useful. By choosing an indication in letters and numbers a classification and grouping can be made. Furthermore, an SOP number makes it possible to refer to the respective procedure with a short notation, for example in Preparation Instructions. |
Date and version number (  ) )A SOP bears a date, usually the date of authorisation, in combination with a version number. Whenever changes in a SOP are made, the version number is incremented. |
Origin/reference (  ) )The source or reason behind the SOP should be recorded and any references included. |
Author/responsible person (  ) )The source or reason behind the SOP should be recorded and any references included. |
Authorisation (  ) )The SOP should be authorised and should bear the signature and function of the authorising person |
Principle/purpose (  ) )It may be helpful to start a SOP with a brief summary, for example when a long or complex process is described. Such a summary is put at the beginning of the text and may contain a brief statement on the purpose of the procedure and its scope. Responsibilities The key responsibilities for the tasks outlined in the SOP may be listed, giving clarity as to the level of staff who carry responsibility at each level. |
Method (  ) )The steps of the procedure preferably should be described as short sentences, with the imperative to create clarity. Where necessary, notes or important background information may be added. It is useful to number each specific point to enable ease of referencing or referral. |
Related SOPs and documents (  ) )This section should list other documents that are referenced within the specific SOP including any related Work Instructions. |
Document history (  ) )Although this may be kept elsewhere in the quality system it is often useful to include a version history of the SOP, summarising the changes made at each version. |
33.4 Batch Preparation Instructions and Records
33.4.1 Definition and Use
Stock preparations are performed according to a standardised preparation instruction, an instruction that has been validated prior to use. This is called a Batch Preparation Instruction (BPI). Also for extemporaneous preparations, a standardised preparation instruction – a BPI – is preferred. However, often the operator has to follow a non-standardised preparation instruction that is less extensive, where no prior validations have been performed, see further Sect. 33.5.
A BPI is developed for a given batch size or a range of batch sizes of a standardised preparation. The formulation has been investigated in advance and the method of preparation has been validated. This means that data are collected and reviewed beforehand to ensure that each batch subsequently prepared will possess the required quality. The preparation method as described in the BPI is therefore only valid for the specified minimum and maximum batch size. The selected batch range depends on the equipment and materials used. Automated preparation documentation systems calculate the quantities to be processed when the batch size is adjusted. In the manual mode, for each batch size within the batch range a separate BPI has to be created. Before the preparation of a specific product is started, whether for the first time or after a change, the responsible pharmacist authorises the BPI and subsequently it is released for use.
The BPI forms the basis of any preparation process. Before starting the preparation of a batch, a copy of the BPI may be made to serve as preparation record during the preparation. The operator uses it to record weighings and measurements during preparation, to record the batch details of the starting materials and to record the results of in process controls and release controls. To distinguish between the original BPI and the copy that is actually used for the preparation of the batch, the copy may be referred to as Batch Preparation Record (BPR).
All the sections of the BPR have to be completed. It is very important that all the information recorded is done in real time. Each raw material should have its batch number recorded ahead of it being measured and the preparers should record their initials as soon as the measurement has been completed to maintain the audit trail. The recording activities for a stock preparation and a standardised extemporaneous preparation are not substantially different, but for extemporaneous preparations a careful accounting of the preparation steps is particularly important, because any subsequent analysis of the product is unlikely.
The preparer has to follow all the specified instructions closely and has to give a written justification for any process deviation, either as planned or unplanned deviations. It is important to follow the instructions exactly, and not to make any unauthorised changes. Uncontrolled changes in batch sizes or quantities of material can lead to serious defects in the final product. For any planned deviation from the prescribed process the reason should be known, and an authorisation by a pharmacist should be given beforehand.
A BPI should be clear and should describe a logical sequence of actions and activities (also called unit operations in manufacturing). For a number of issues it may refer to general SOPs or WIs.
For basic operations, the BPI may refer to a SOP, for example for the process of the filling of capsules.
For the use and cleaning of specific equipment the BPI may refer to WIs.
For controls the BPI may refer to a SOP, e.g. for sampling.
For analytical testing, the BPI may refer to a separate analysis instruction designed to test the preparation or the specific dosage form. When analysis is outsourced, the BPI may mention that a sample should be sent to the approved laboratory.
In the BPI only those quality specifications are included which can be tested during or immediately after preparation. An overview of requirements and analytical tests for pharmacy preparations can be found in Table 32.2.
33.4.2 Drafting a Batch Preparation Instruction
When a BPI is drafted, it is important to present a clear, compact collection of information. The text must be unambiguous, legible and factually correct. Depending on the scale of production, a BPI may consist of only one page for a standardised extemporaneous preparation or may contain multiple pages for stock preparations. The right combination of preparation process, in-process controls and release controls should guarantee the quality of the preparation.
Obviously, the BPI should be kept clear for proper use and proper control. Therefore, it is important to mention only the issues that are directly related to the preparation of the product. When drafting a BPI some editorial guidance can be given:
Sentence Structures:
Choose the imperative.
Use short sentences, with proper punctuation.
Describe, if possible, one operation (unit operation) per sentence.
Arrange the operations in chronological order. So for example: ‘Dissolve X in Y. Triturate Z with this solution’ instead of ‘Triturate Z with a solution of X in Y’.
Wording:
Use familiar words.
Avoid composed words.
Avoid ‘heavy’ expressions, for example, rather ‘with’ instead of ‘with the aid of’.
Sometimes it is possible to use a general exemplary BPI belonging to a standard formulation (such as with FNA or NRF). Such an exemplary BPI will be of a standard template and has to be adapted to the local pharmacy situation. The description of the preparation method may have to be adapted regarding the batch size and the available utensils and equipment in the specific pharmacy situation. For general preparation methods, e.g. for filling of capsules or for the folding of tubes, the general SOP that describes the appropriate preparation method should be referenced.
In larger preparation units, for example the hospital pharmacy, often an automated system for the drafting of BPIs is used. Within such a system, it is usually possible to create one or more standard layouts, adjusted to the needs of the specific organisation and adapted to the type of preparation or the pharmaceutical form.
The sections of the BPI are described in Fig. 33.3, which shows an example of a Batch Preparation Instruction. The items of the BPI are mentioned below. The numbers (if any) correspond to the sections of both Figs. 33.3 and 33.5.
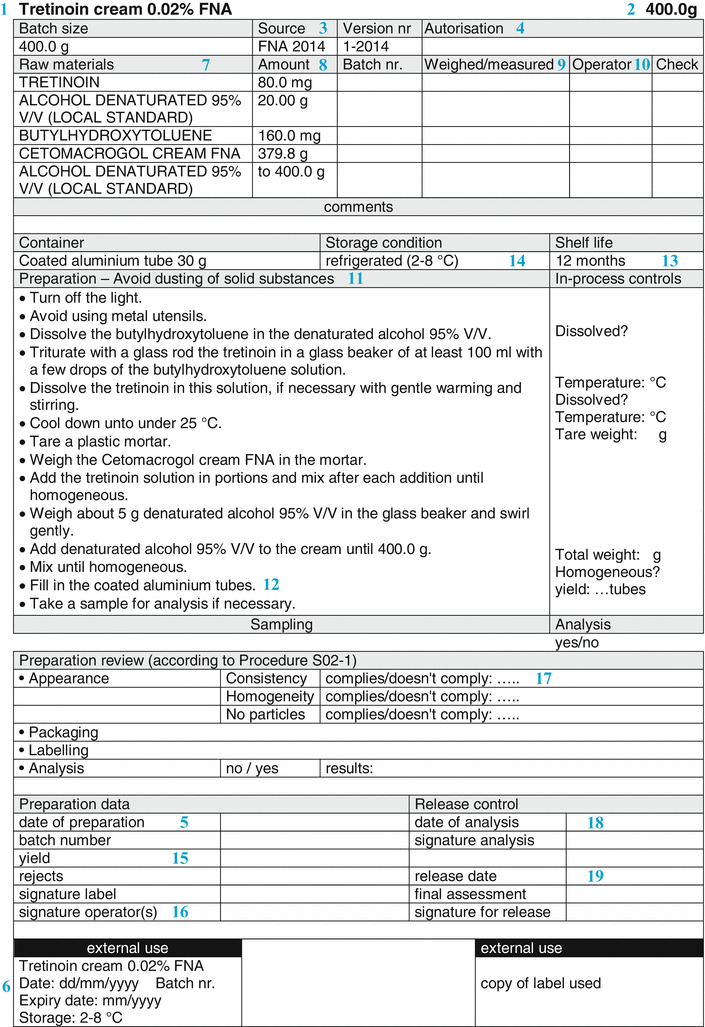

Fig. 33.3
Example of a batch preparation instruction
Name, dosage form and strength of the preparation ( 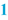 ) )The name of the preparation is usually the title of the BPI. The name of the preparation normally includes the strength of the active substance. When the preparation contains more than one active substance, it may be decided to assign a specific name to the preparation. It may also be necessary to include the batch size (see below) in this title area where a variety of BPIs exist for a specific product formulation. |
Batch Size ( 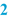 ) )Subsequently, usually the batch size or batch range is mentioned. The latter means that the minimum and maximum batch size are defined, resulting in a more flexible BPI. The batch size can be specified as follows: • Number (for suppositories, capsules); • Weight (for ointments, creams); • Volume (for oral solutions, lotions); • Volume plus weight between brackets (for solutions with a density unequal to 1); |
• Volume plus number of containers between brackets (e.g. eye drops). |
Origin/reference ( 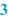 ) )Also, it may be useful to refer to the origin of the formulation. This may be a formulary, a scientific publication, information from a manufacturer (e.g. about products that have been withdrawn from the market), or a reference to a proprietary formulation or one from a colleague. It may be useful to mention a version number or date of publication, to give a proper reference. |
Authorisation and date of creation ( 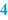 ) )The person responsible for the content and implementation of the BPI, usually the pharmacist, places his signature of approval initials to authorise the document. By authorising it, the BPI is released for use. An unauthorised BPI should never be used. Automated systems are normally equipped with a versioning option. In that case the pharmacist authorises the BPI in the computer. Only when this has been done, can the BPI be used for preparation. When the BPI is modified, the authorisation automatically expires and it cannot be used until the new version is authorised. Together with the authorisation usually the date of creation or modification is mentioned. A version number on the BPI may serve the same purpose. In this way, it will be possible to check if the latest version is used. In automated systems, the system usually records the version number and date of modification automatically. |
Batch number and Preparation date ( 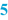 ) )A BPI normally leaves some blank space, where the batch number of the preparation and the date of its preparation can be noted during preparation. For a standard extemporaneous preparation the prescription number can be added for traceability. In order to limit the amount of text, the preparation date and batch number can be combined in one number. For example, when the date is mentioned as YYMMDD, an ascending numbering is obtained, which is simply traceable to the date. For larger scale production, an extended batch number is needed for distinguishing different preparations and to obtain a unique batch numbering. It is common to add a number to the date, for example in the form of: 141015-001, 141015-002, 141015-003, etc. Also it is possible to use an alphabetical coding of the months, starting with A for January, B for February and so on. For any system chosen, the important point is that the traceability of each batch is guaranteed. |
Reference label ( 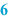 ) )Normally, a BPI is provided with a reference label for the label to be used. On a paper BPI the reference label can be stuck directly, in an automated system the label data may be recorded in the master label template. All the relevant data for the preparation should be included on the master label, only variable data fields such as batch number and expiration date should be left blank. According to [2] the following information should be included on the label: • Name, dosage form and strength of the preparation • Full qualitative composition and the quantity of the active substance • Batch number • Expiry date • Special storage conditions or handling precautions • Directions for use, warnings and precautions • Route of administration See Sect. 37.3.2 for additional information on labelling for the patient. It is useful to leave some space on the BPI where a copy of the label that will be used for the batch concerned can be attached. Ideally this should be next to the sample label. |
Raw materials and packaging materials ( 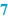 ) )Subsequently, the BPI provides a list of all active substances and excipients, in a way that defines their identity and quality unambiguously. For example, it should be clear if the free active substance has to be used or the salt form, or, when multiple hydrates exist, which hydrated form is required (see Sect. 23.1).To define the quality of the raw materials it is advisable to use their pharmacopoeial names wherever possible. Preferably, additional specifications (Functional related characteristics, FRCs) which are not mentioned in the monograph that distinguish between different qualities, are added to the name of the raw material. Examples are the particle size of a solid material, the viscosity of a liquid or a cellulose derivative, or the concentration of a solution. Sometimes a brand name reflects the quality better (e.g. Witepsol H15 instead of Adeps solidus). If confusion may still be possible (crystal water, salt forms), addition of the chemical formula might be useful. It is also important to include a description of the containers, including size and quantity and if necessary other quality characteristics. Raw materials and containers are mentioned in the same order in which the operator will use them. The reason for this is to help the operator record the batch numbers and signatures on the correct line of the BPR. In automated preparation systems, raw materials usually have to be used in the listed order. Their sequence may depend on the equipment used or the batch size. The BPI should have space where the identity of the raw materials and packaging materials that have actually been used may be recorded, for example by their batch numbers. This may be done either manually or by an automated system with a barcode reader. |
Quantity ( 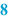 ) )For each raw material that should be weighed or measured, the needed quantity is listed on the BPI. Excipients which are required on more than one occasion during preparation, may be mentioned separately each time they are needed in the required amount. Also the quantities of the required packaging materials are listed, such as the number of suppository strips. The quantities listed are given to the accuracy that they need to be measured (see Sect. 29.1). It is advisable to specify the balance to be used in the BPI. This ensures the use of a balance with sufficient accuracy for the weighing process. All amounts are mentioned followed by their units, for example grams (g) or milliliters (mL). Weighing is to be preferred (see Sect. 29.1.2) but it may be simpler to work with volumes rather than weights. In that case adequate controls must be in place to ensure that the right volumes have been added. Generally weighing devices can give print-outs of weighings made but volume devices cannot. |
Weighed/measured ( 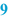 ) )The list of raw materials on the BPI is usually followed by spaces where the operator records the actual quantities which are weighed or measured, including the units. This may be done manually, but the use of an automated system coupled with an electronic balance provides a better opportunity to capture the weighed quantities. With a manual system there should still be a printed record of any weighings made. |
Initials and control (  ) )There is generally space where the preparer can place his initials for each measurement or weighing. During preparation, for each raw material the label on the container is compared with the prescribed material on the BPI, then the source and the batch number are recorded, and the material is weighed or measured. The measured quantity is recorded, the raw material is processed and the preparer places his initials. On the BPI model (Fig. 33.2) a column is reserved for the initials of the operator, marked with the letter ‘O’. There may also be space for the initials of a second employee, who checks during preparation each weighing or volume measurement. In automated system, the weights are controlled and printed automatically. The validation of the system is a prerequisite. Simple weighing systems cannot monitor the volumes measured, which requires a check by a second employee. Whatever process is used, the measurements and checks must be verifiable. Obviously, a print out of the balance may be sufficient. It can be attached to the preparation record by the preparer, but it should be understood that this print out alone does not prove which material was weighed. It may be that a system such as barcode scanning needs to be used alongside it if the second check at the time of preparation is to be omitted. |
Preparation method (  ) )The BPI has to provide a clear description of the preparation method, including in process controls and line clearances. All preparation operations are described step-by-step (as unit operations. To improve the readability each step best starts on a new line. If not, all unit operations may be marked separately, to make them easily recognisable. In any case, the essential points of the applied method of preparation, including all in process-controls as well as occupational safety aspects have to be listed. Also precautions should be stated to prevent any hazards such as fire or damage by corrosive properties of substances. |
Packaging process; (  ) )A description of the packaging process in a separate part of the preparation instruction may be added. |
In-process controls (  ) )In process controls that the operator runs during production are mentioned separately on the BPI. In the BPI model a separate section is reserved for these in-process controls. When applicable these controls are described on the same line as the preparation step to which they apply. In that way the operator is instructed to perform the control immediately after this step. In the description of the preparation, the control moments can be emphasised by mentioning them as ‘check now …’ or ‘record now … “. This should encourage the operator to perform the control at that moment, and not at the end of the preparation. When working in a specific environment, such as laminar air flow (LAF) cabinet, the preparer cannot always interrupt his work to place his initials on a paper record as this would represent poor aseptic practice. In that particular case, a second employee may complete the BPR, and any required signatures can be completed at the end of the process. However, there are also LAF cabinets with built-in display, where the preparer can place his initials electronically. Automated systems may have obligatory in-process controls, which require that the operator records a result before he is able to proceed to the next step. The nature and extent of the in-process controls are determined in conjunction with the controls on the raw materials before, and in conjunction with the release controls which will follow afterwards. Together these controls ensure the quality of the final product. If the preparer has to perform a select sampling during preparation, for example to check the content distribution of capsules or suppositories, the sampling method should be described in the preparation text and not under the release controls of the final product. Also the weighings used for a weight control of capsules or suppositories should be mentioned in the preparation text and are part of the preparation process. |
Equipment
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|