PYROGENS
The term pyrogen (Greek pyro, “fire”) is used to describe any substance that causes fever. Exogenous pyrogens are derived from outside the patient; most are microbial products, microbial toxins, or whole microorganisms (including viruses). The classic example of an exogenous pyrogen is the lipopolysaccharide (endotoxin) produced by all gram-negative bacteria. Pyrogenic products of gram-positive organisms include the enterotoxins of Staphylococcus aureus and the groups A and B streptococcal toxins, also called superantigens. One staphylococcal toxin of clinical importance is that associated with isolates of S. aureus from patients with toxic shock syndrome. These products of staphylococci and streptococci cause fever in experimental animals when injected intravenously at concentrations of 1–10 μg/kg. Endotoxin is a highly pyrogenic molecule in humans: when injected intravenously into volunteers, a dose of 2–3 ng/kg produces fever, leukocytosis, acute-phase proteins, and generalized symptoms of malaise.
PYROGENIC CYTOKINES
Cytokines are small proteins (molecular mass, 10,000–20,000 Da) that regulate immune, inflammatory, and hematopoietic processes. For example, the elevated leukocytosis seen in several infections with an absolute neutrophilia is attributable to the cytokines interleukin (IL) 1 and IL-6. Some cytokines also cause fever; formerly referred to as endogenous pyrogens, they are now called pyrogenic cytokines. The pyrogenic cytokines include IL-1, IL-6, tumor necrosis factor (TNF), and ciliary neurotropic factor, a member of the IL-6 family. Interferons (IFNs), particularly IFN-α, also are pyrogenic cytokines; fever is a prominent side effect of IFN-α used in the treatment of hepatitis. Each pyrogenic cytokine is encoded by a separate gene, and each has been shown to cause fever in laboratory animals and in humans. When injected into humans at low doses (10–100 ng/kg), IL-1 and TNF produce fever; in contrast, for IL-6, a dose of 1–10 μg/kg is required for fever production.
A wide spectrum of bacterial and fungal products induce the synthesis and release of pyrogenic cytokines. However, fever can be a manifestation of disease in the absence of microbial infection. For example, inflammatory processes, trauma, tissue necrosis, and antigen-antibody complexes induce the production of IL-1, TNF, and/or IL-6; individually or in combination, these cytokines trigger the hypothalamus to raise the set point to febrile levels.
ELEVATION OF THE HYPOTHALAMIC SET POINT BY CYTOKINES
During fever, levels of prostaglandin E2 (PGE2) are elevated in hypothalamic tissue and the third cerebral ventricle. The concentrations of PGE2 are highest near the circumventricular vascular organs (organum vasculosum of lamina terminalis)—networks of enlarged capillaries surrounding the hypothalamic regulatory centers. Destruction of these organs reduces the ability of pyrogens to produce fever. Most studies in animals have failed to show, however, that pyrogenic cytokines pass from the circulation into the brain itself. Thus, it appears that both exogenous pyrogens and pyrogenic cytokines interact with the endothelium of these capillaries and that this interaction is the first step in initiating fever—i.e., in raising the set point to febrile levels.
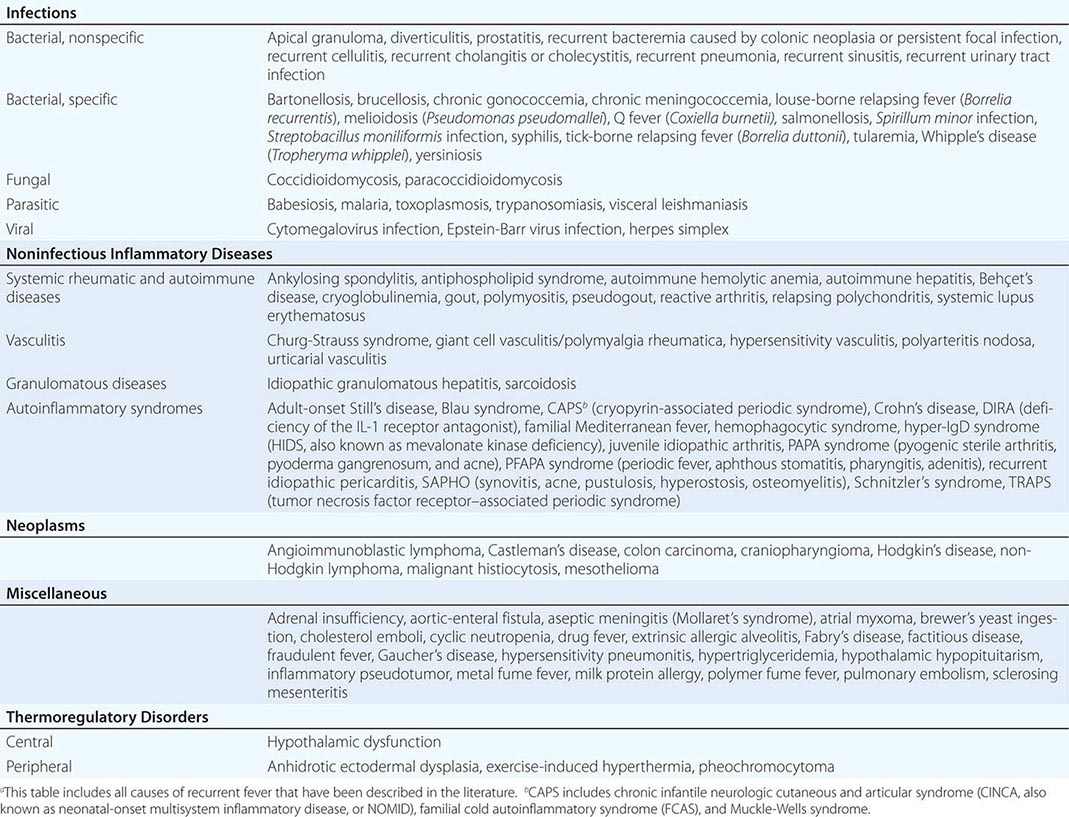
The key events in the production of fever are illustrated in Fig. 23-1. Myeloid and endothelial cells are the primary cell types that produce pyrogenic cytokines. Pyrogenic cytokines such as IL-1, IL-6, and TNF are released from these cells and enter the systemic circulation. Although these circulating cytokines lead to fever by inducing the synthesis of PGE2, they also induce PGE2 in peripheral tissues. The increase in PGE2 in the periphery accounts for the nonspecific myalgias and arthralgias that often accompany fever. It is thought that some systemic PGE2 escapes destruction by the lung and gains access to the hypothalamus via the internal carotid. However, it is the elevation of PGE2 in the brain that starts the process of raising the hypothalamic set point for core temperature.
FIGURE 23-1 Chronology of events required for the induction of fever. AMP, adenosine 5′-monophosphate; IFN, interferon; IL, interleukin; PGE2, prostaglandin E2; TNF, tumor necrosis factor.
There are four receptors for PGE2, and each signals the cell in different ways. Of the four receptors, the third (EP-3) is essential for fever: when the gene for this receptor is deleted in mice, no fever follows the injection of IL-1 or endotoxin. Deletion of the other PGE2 receptor genes leaves the fever mechanism intact. Although PGE2 is essential for fever, it is not a neurotransmitter. Rather, the release of PGE2 from the brain side of the hypothalamic endothelium triggers the PGE2 receptor on glial cells, and this stimulation results in the rapid release of cyclic adenosine 5′-monophosphate (cAMP), which is a neurotransmitter. As shown in Fig. 23-1, the release of cAMP from glial cells activates neuronal endings from the thermoregulatory center that extend into the area. The elevation of cAMP is thought to account for changes in the hypothalamic set point either directly or indirectly (by inducing the release of neurotransmitters). Distinct receptors for microbial products are located on the hypothalamic endothelium. These receptors are called Toll-like receptors and are similar in many ways to IL-1 receptors. IL-1 receptors and Toll-like receptors share the same signal-transducing mechanism. Thus, the direct activation of Toll-like receptors or IL-1 receptors results in PGE2 production and fever.
PRODUCTION OF CYTOKINES IN THE CNS
Cytokines produced in the brain may account for the hyperpyrexia of CNS hemorrhage, trauma, or infection. Viral infections of the CNS induce microglial and possibly neuronal production of IL-1, TNF, and IL-6. In experimental animals, the concentration of a cytokine required to cause fever is several orders of magnitude lower with direct injection into the brain substance or brain ventricles than with systemic injection. Therefore, cytokines produced in the CNS can raise the hypothalamic set point, bypassing the circumventricular organs. CNS cytokines likely account for the hyperpyrexia of CNS hemorrhage, trauma, or infection.
24 | Fever and Rash |
The acutely ill patient with fever and rash may present a diagnostic challenge for physicians. However, the distinctive appearance of an eruption in concert with a clinical syndrome can facilitate a prompt diagnosis and the institution of life-saving therapy or critical infection-control interventions. Representative images of many of the rashes discussed in this chapter are included in Chap. 25e.
For further discussion, see Chaps. 70, 72, and 147.
CLASSIFICATION OF RASH
This chapter reviews rashes that reflect systemic disease, but it does not include localized skin eruptions (i.e., cellulitis, impetigo) that may also be associated with fever (Chap. 156). The chapter is not intended to be all-inclusive, but it covers the most important and most common diseases associated with fever and rash. Rashes are classified herein on the basis of lesion morphology and distribution. For practical purposes, this classification system is based on the most typical disease presentations. However, morphology may vary as rashes evolve, and the presentation of diseases with rashes is subject to many variations (Chap. 72). For instance, the classic petechial rash of Rocky Mountain spotted fever (Chap. 211) may initially consist of blanchable erythematous macules distributed peripherally; at times, however, the rash associated with this disease may not be predominantly acral, or no rash may develop at all.
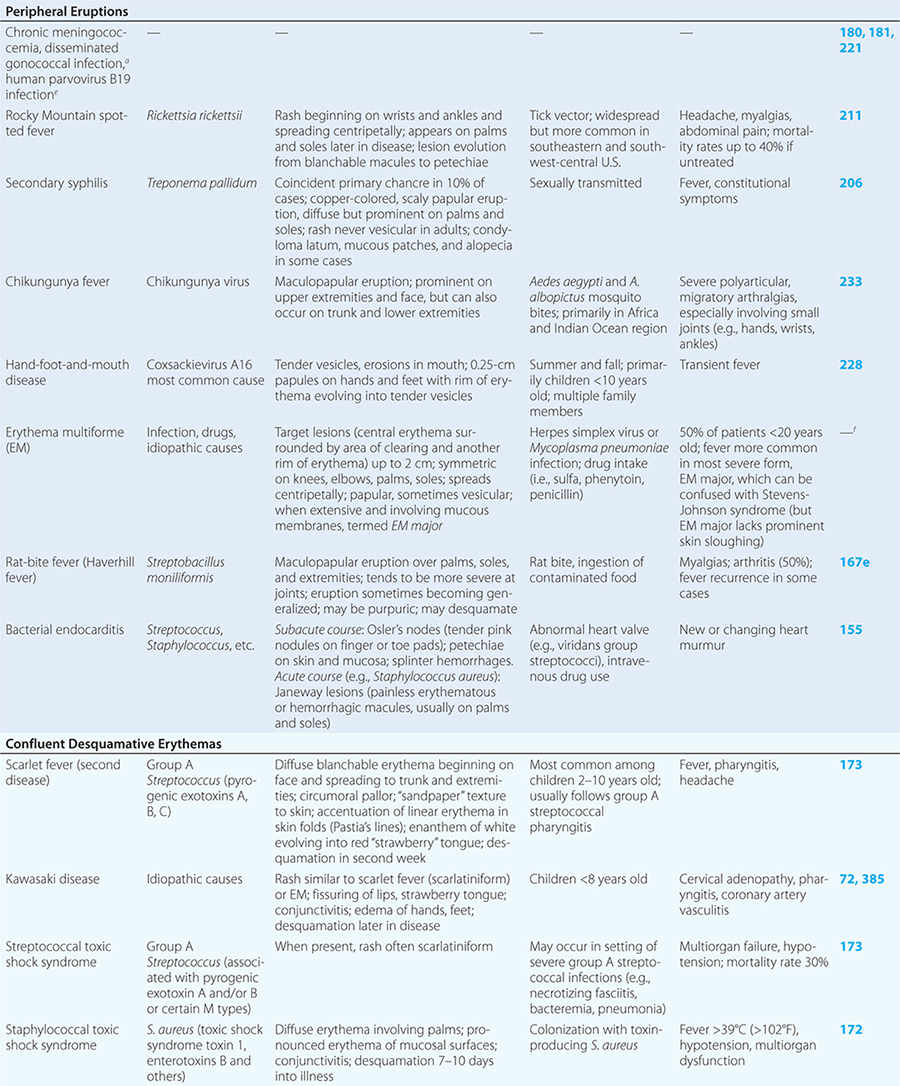
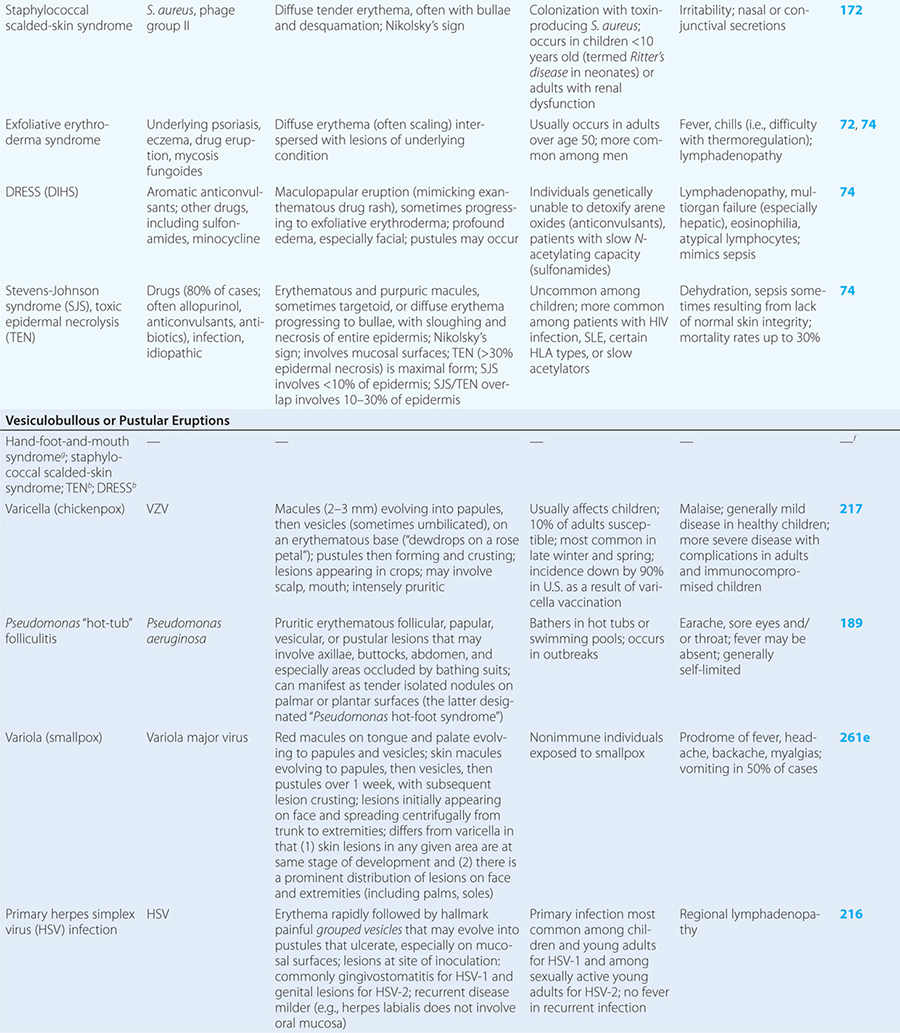
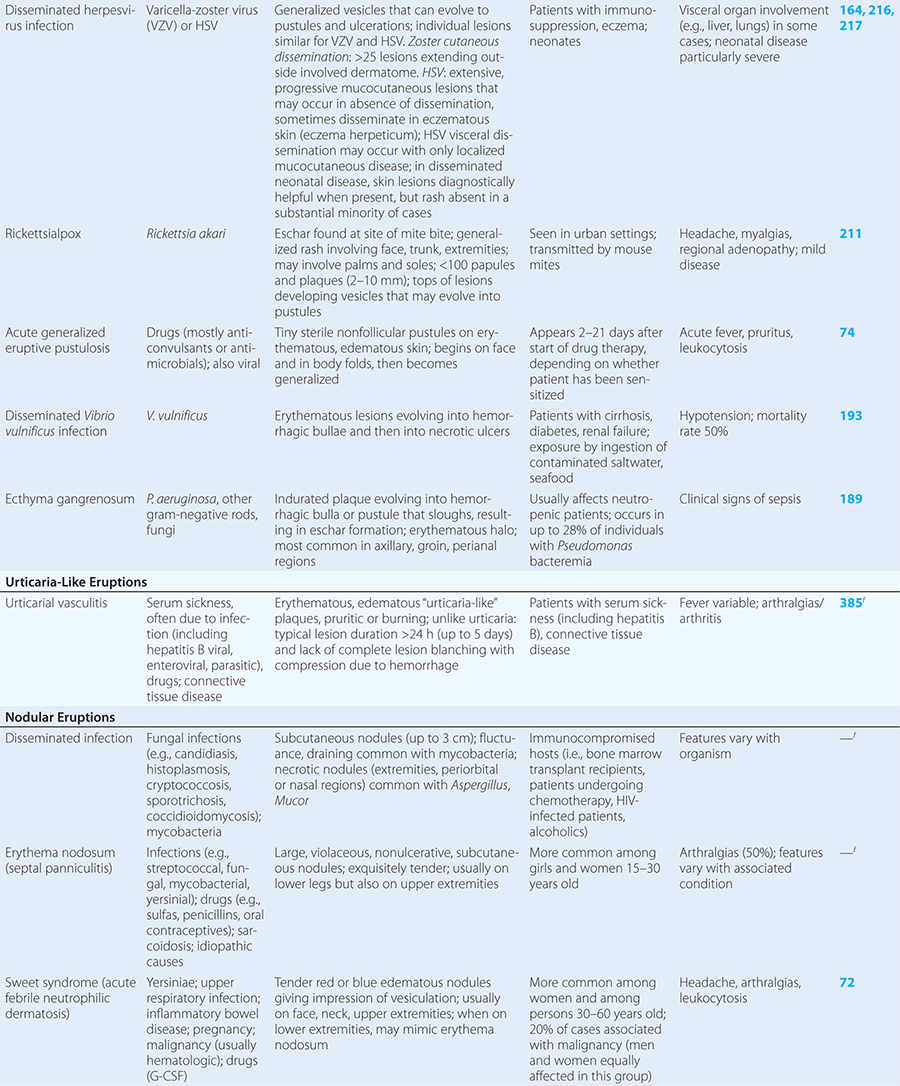
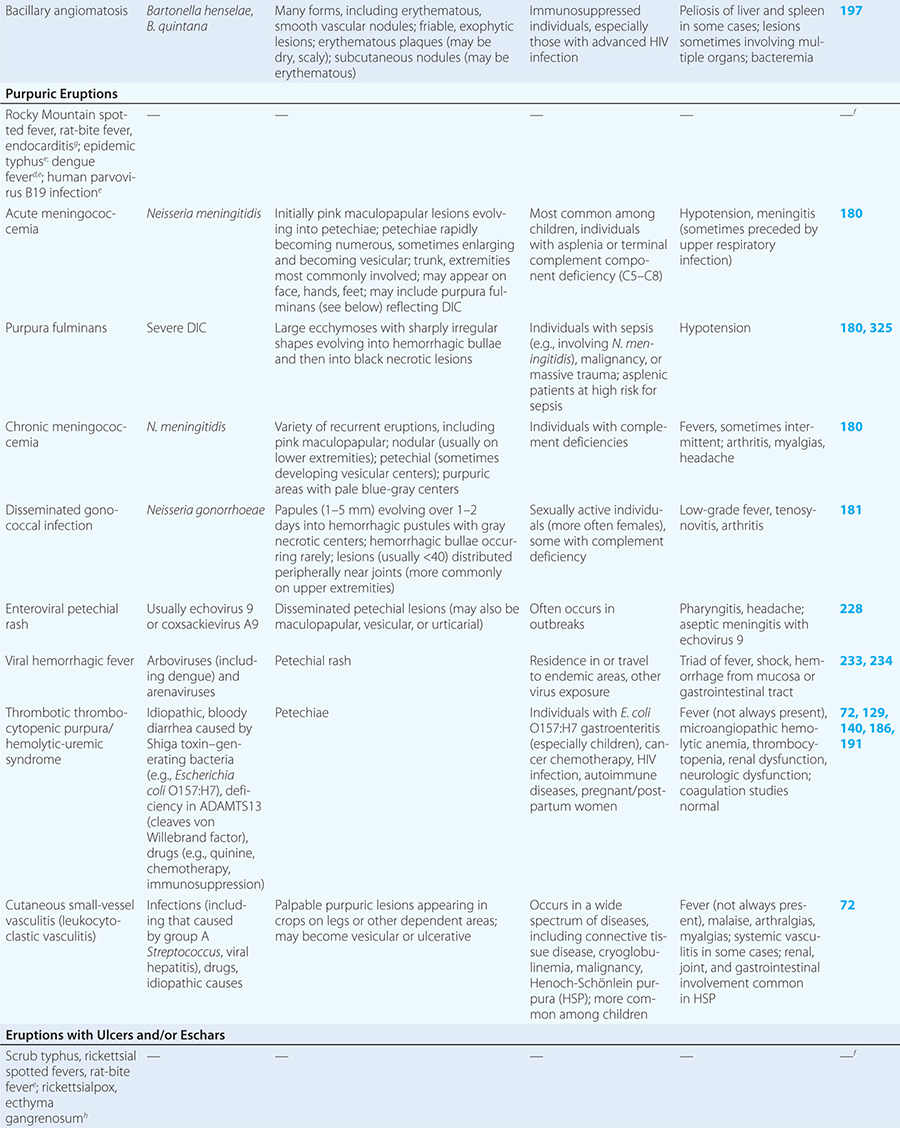
Diseases with fever and rash may be classified by type of eruption: centrally distributed maculopapular, peripheral, confluent desquamative erythematous, vesiculobullous, urticaria-like, nodular, purpuric, ulcerated, or with eschars. Diseases are listed by these categories in Table 24-1, and many are highlighted in the text. However, for a more detailed discussion of each disease associated with a rash, the reader is referred to the chapter dealing with that specific disease. (Reference chapters are cited in the text and listed in Table 24-1.)
DISEASES ASSOCIATED WITH FEVER AND RASH |

CENTRALLY DISTRIBUTED MACULOPAPULAR ERUPTIONS
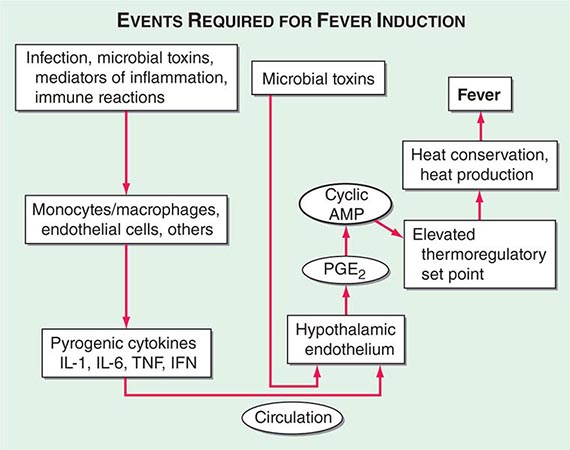
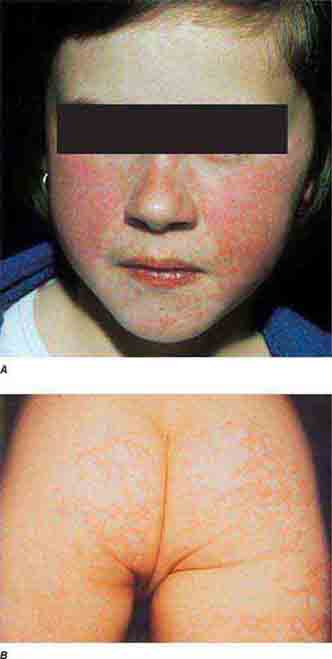
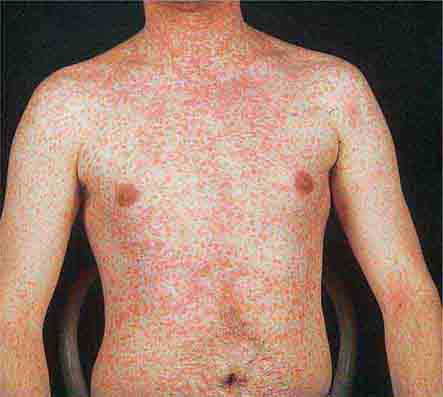
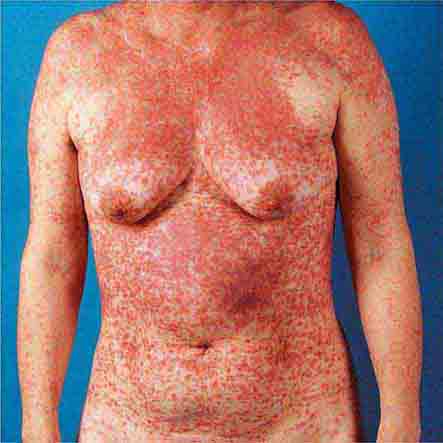
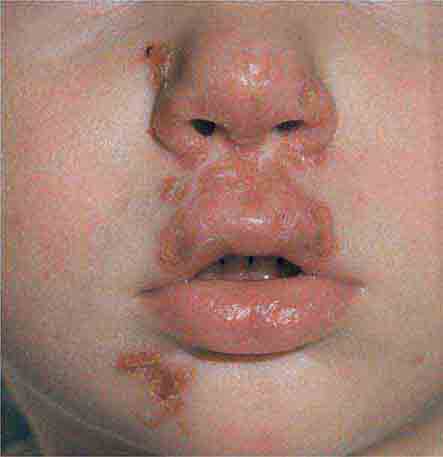
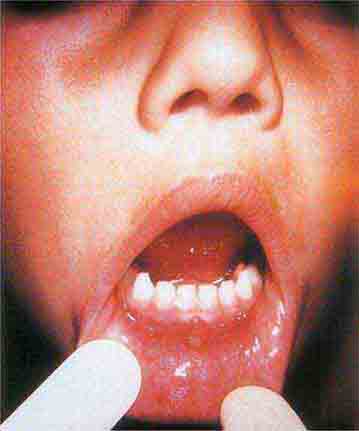
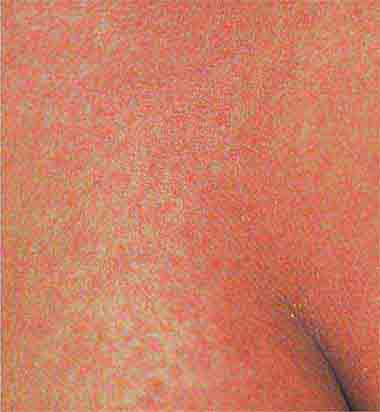
Centrally distributed rashes, in which lesions are primarily truncal, are the most common type of eruption. The rash of rubeola (measles) starts at the hairline 2–3 days into the illness and moves down the body, typically sparing the palms and soles (Chap. 229). It begins as discrete erythematous lesions, which become confluent as the rash spreads. Koplik’s spots (1- to 2-mm white or bluish lesions with an erythematous halo on the buccal mucosa) are pathognomonic for measles and are generally seen during the first 2 days of symptoms. They should not be confused with Fordyce’s spots (ectopic sebaceous glands), which have no erythematous halos and are found in the mouth of healthy individuals. Koplik’s spots may briefly overlap with the measles exanthem.
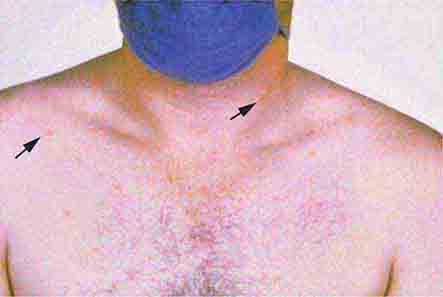
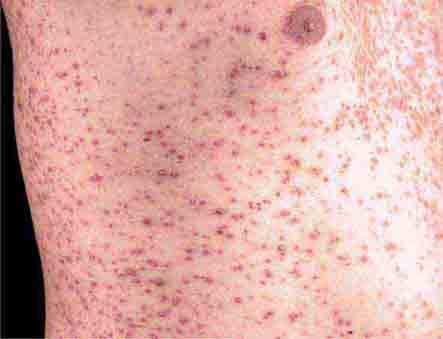
Rubella (German measles) also spreads from the hairline downward; unlike that of measles, however, the rash of rubella tends to clear from originally affected areas as it migrates, and it may be pruritic (Chap. 230e). Forchheimer spots (palatal petechiae) may develop but are nonspecific because they also develop in infectious mononucleosis (Chap. 218) and scarlet fever (Chap. 173). Postauricular and suboccipital adenopathy and arthritis are common among adults with rubella. Exposure of pregnant women to ill individuals should be avoided, as rubella causes severe congenital abnormalities. Numerous strains of enteroviruses (Chap. 228), primarily echoviruses and coxsackieviruses, cause nonspecific syndromes of fever and eruptions that may mimic rubella or measles. Patients with infectious mononucleosis caused by Epstein-Barr virus (Chap. 218) or with primary HIV infection (Chap. 226) may exhibit pharyngitis, lymphadenopathy, and a nonspecific maculopapular exanthem.
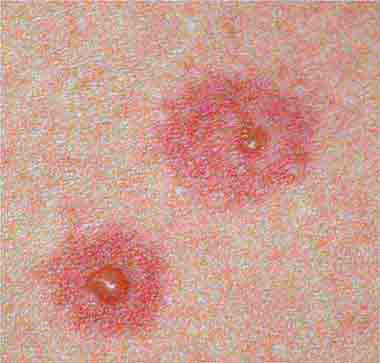
The rash of erythema infectiosum (fifth disease), which is caused by human parvovirus B19, primarily affects children 3–12 years old; it develops after fever has resolved as a bright blanchable erythema on the cheeks (“slapped cheeks”) with perioral pallor (Chap. 221). A more diffuse rash (often pruritic) appears the next day on the trunk and extremities and then rapidly develops into a lacy reticular eruption that may wax and wane (especially with temperature change) over 3 weeks. Adults with fifth disease often have arthritis, and fetal hydrops can develop in association with this condition in pregnant women.
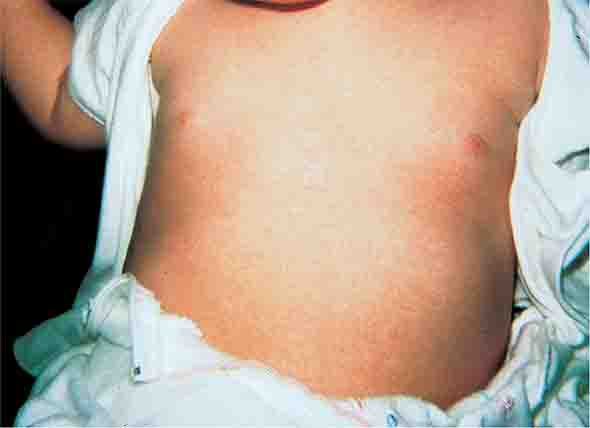
Exanthem subitum (roseola) is caused by human herpesvirus 6 and is most common among children <3 years of age (Chap. 219). As in erythema infectiosum, the rash usually appears after fever has subsided. It consists of 2- to 3-mm rose-pink macules and papules that coalesce only rarely, occur initially on the trunk and sometimes on the extremities (sparing the face), and fade within 2 days.
Although drug reactions have many manifestations, including urticaria, exanthematous drug-induced eruptions (Chap. 74) are most common and are often difficult to distinguish from viral exanthems. Eruptions elicited by drugs are usually more intensely erythematous and pruritic than viral exanthems, but this distinction is not reliable. A history of new medications and an absence of prostration may help to distinguish a drug-related rash from an eruption of another etiology. Rashes may persist for up to two weeks after administration of the offending agent is discontinued. Certain populations are more prone than others to drug rashes. Of HIV-infected patients, 50–60% develop a rash in response to sulfa drugs; 90% of patients with mononucleosis due to Epstein-Barr virus develop a rash when given ampicillin.
Rickettsial illnesses (Chap. 211) should be considered in the evaluation of individuals with centrally distributed maculopapular eruptions. The usual setting for epidemic typhus is a site of war or natural disaster in which people are exposed to body lice. Endemic typhus or leptospirosis (the latter caused by a spirochete) (Chap. 208) may be seen in urban environments where rodents proliferate. Outside the United States, other rickettsial diseases cause a spotted-fever syndrome and should be considered in residents of or travelers to endemic areas. Similarly, typhoid fever, a nonrickettsial disease caused by Salmonella typhi (Chap. 190), is usually acquired during travel outside the United States. Dengue fever, caused by a mosquito-transmitted flavivirus, occurs in tropical and subtropical regions of the world (Chap. 233).
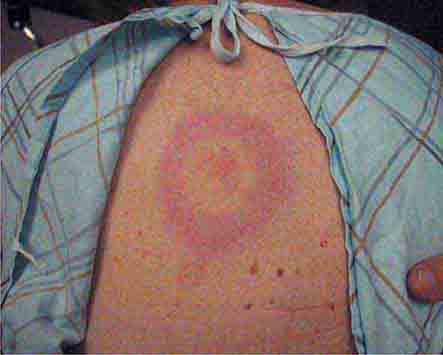
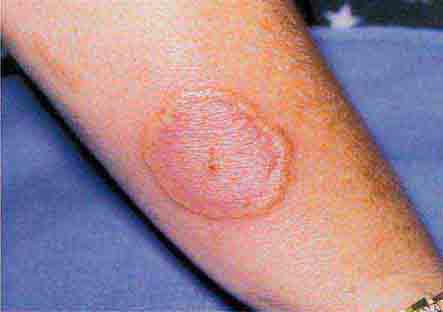
Some centrally distributed maculopapular eruptions have distinctive features. Erythema migrans, the rash of Lyme disease (Chap. 210), typically manifests as single or multiple annular plaques. Untreated erythema migrans lesions usually fade within a month but may persist for more than a year. Southern tick-associated rash illness (STARI) (Chap. 210) has an erythema migrans–like rash but is less severe than Lyme disease and often occurs in regions where Lyme is not endemic. Erythema marginatum, the rash of acute rheumatic fever (Chap. 381), has a distinctive pattern of enlarging and shifting transient annular lesions.
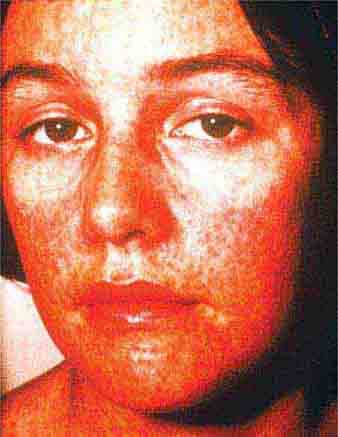
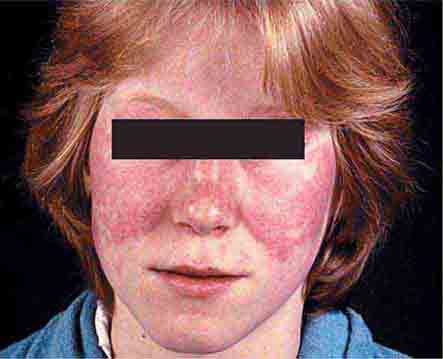
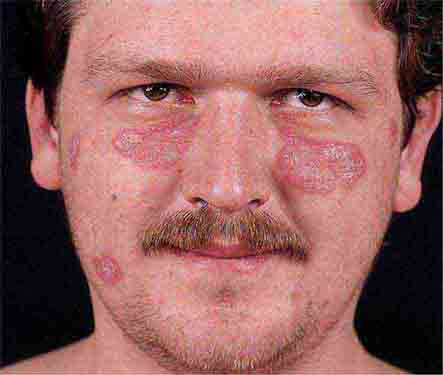
Collagen vascular diseases may cause fever and rash. Patients with systemic lupus erythematosus (Chap. 378) typically develop a sharply defined, erythematous eruption in a butterfly distribution on the cheeks (malar rash) as well as many other skin manifestations. Still’s disease (Chap. 398) presents as an evanescent, salmon-colored rash on the trunk and proximal extremities that coincides with fever spikes.
PERIPHERAL ERUPTIONS
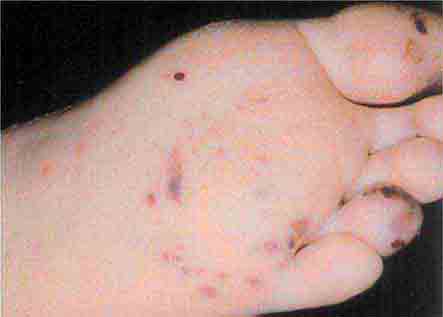
These rashes are alike in that they are most prominent peripherally or begin in peripheral (acral) areas before spreading centripetally. Early diagnosis and therapy are critical in Rocky Mountain spotted fever (Chap. 211) because of its grave prognosis if untreated. Lesions evolve from macular to petechial, start on the wrists and ankles, spread centripetally, and appear on the palms and soles only later in the disease. The rash of secondary syphilis (Chap. 206), which may be generalized but is prominent on the palms and soles, should be considered in the differential diagnosis of pityriasis rosea, especially in sexually active patients. Chikungunya fever (Chap. 233), which is transmitted by mosquito bite in Africa and the Indian Ocean region, is associated with a maculopapular eruption and severe polyarticular small-joint arthralgias. Hand-foot-and-mouth disease (Chap. 228), most commonly caused by coxsackievirus A16, is distinguished by tender vesicles distributed peripherally and in the mouth; outbreaks commonly occur within families. The classic target lesions of erythema multiforme appear symmetrically on the elbows, knees, palms, soles, and face. In severe cases, these lesions spread diffusely and involve mucosal surfaces. Lesions may develop on the hands and feet in endocarditis (Chap. 155).
CONFLUENT DESQUAMATIVE ERYTHEMAS
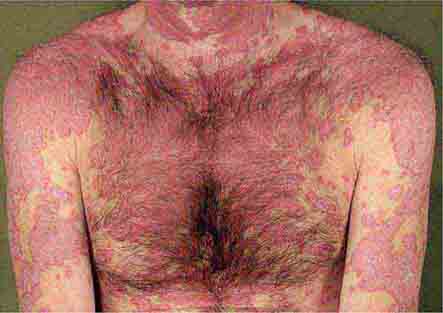
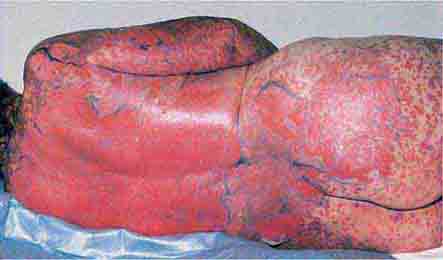
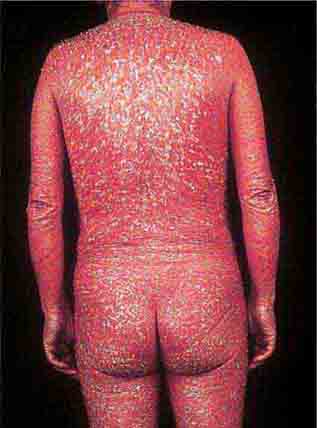
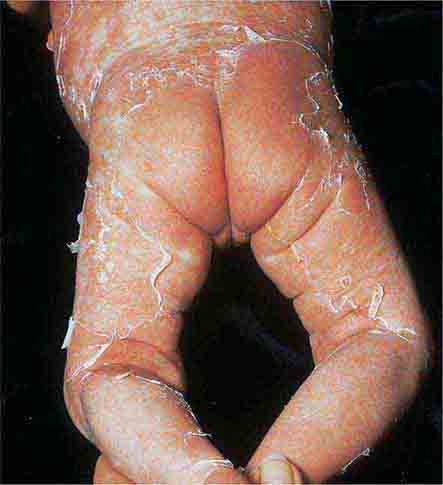
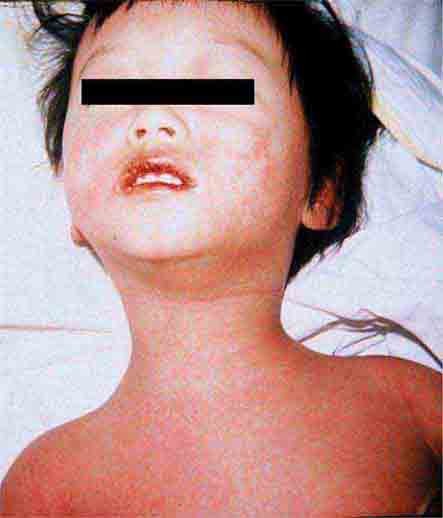
These eruptions consist of diffuse erythema frequently followed by desquamation. The eruptions caused by group A Streptococcus or Staphylococcus aureus are toxin-mediated. Scarlet fever (Chap. 173) usually follows pharyngitis; patients have a facial flush, a “strawberry” tongue, and accentuated petechiae in body folds (Pastia’s lines). Kawasaki disease (Chaps. 72 and 385) presents in the pediatric population as fissuring of the lips, a strawberry tongue, conjunctivitis, adenopathy, and sometimes cardiac abnormalities. Streptococcal toxic shock syndrome (Chap. 173) manifests with hypotension, multiorgan failure, and, often, a severe group A streptococcal infection (e.g., necrotizing fasciitis). Staphylococcal toxic shock syndrome (Chap. 172) also presents with hypotension and multiorgan failure, but usually only S. aureus colonization—not a severe S. aureus infection—is documented. Staphylococcal scalded-skin syndrome (Chap. 172) is seen primarily in children and in immunocompromised adults. Generalized erythema is often evident during the prodrome of fever and malaise; profound tenderness of the skin is distinctive. In the exfoliative stage, the skin can be induced to form bullae with light lateral pressure (Nikolsky’s sign). In a mild form, a scarlatiniform eruption mimics scarlet fever, but the patient does not exhibit a strawberry tongue or circumoral pallor. In contrast to the staphylococcal scalded-skin syndrome, in which the cleavage plane is superficial in the epidermis, toxic epidermal necrolysis (Chap. 74), a maximal variant of Stevens-Johnson syndrome, involves sloughing of the entire epidermis, resulting in severe disease. Exfoliative erythroderma syndrome (Chaps. 72 and 74) is a serious reaction associated with systemic toxicity that is often due to eczema, psoriasis, a drug reaction, or mycosis fungoides. Drug rash with eosinophilia and systemic symptoms (DRESS), often due to antiepileptic and antibiotic agents (Chap. 74), initially appears similar to an exanthematous drug reaction but may progress to exfoliative erythroderma; it is accompanied by multi-organ failure and has an associated mortality rate of ~10%.
VESICULOBULLOUS OR PUSTULAR ERUPTIONS
Varicella (Chap. 217) is highly contagious, often occurring in winter or spring. At any point in time, within a given region of the body, varicella lesions are in different stages of development. In immunocompromised hosts, varicella vesicles may lack the characteristic erythematous base or may appear hemorrhagic. Lesions of Pseudomonas “hot-tub” folliculitis (Chap. 189) are also pruritic and may appear similar to those of varicella. However, hot-tub folliculitis generally occurs in outbreaks after bathing in hot tubs or swimming pools, and lesions occur in regions occluded by bathing suits. Lesions of variola (smallpox) (Chap. 261e) also appear similar to those of varicella but are all at the same stage of development in a given region of the body. Variola lesions are most prominent on the face and extremities, while varicella lesions are most prominent on the trunk. Herpes simplex virus infection (Chap. 216) is characterized by hallmark grouped vesicles on an erythematous base. Primary herpes infection is accompanied by fever and toxicity, while recurrent disease is milder. Rickettsialpox (Chap. 211) is often documented in urban settings and is characterized by vesicles followed by pustules. It can be distinguished from varicella by an eschar at the site of the mouse-mite bite and the papule/plaque base of each vesicle. Acute generalized eruptive pustulosis should be considered in individuals who are acutely febrile and are taking new medications, especially anticonvulsant or antimicrobial agents (Chap. 74). Disseminated Vibrio vulnificus infection (Chap. 193) or ecthyma gangrenosum due to Pseudomonas aeruginosa (Chap. 189) should be considered in immunosuppressed individuals with sepsis and hemorrhagic bullae.
URTICARIA-LIKE ERUPTIONS
Individuals with classic urticaria (“hives”) usually have a hypersensitivity reaction without associated fever. In the presence of fever, urticaria-like eruptions are most often due to urticarial vasculitis (Chap. 385). Unlike individual lesions of classic urticaria, which last up to 24 h, these lesions may last 3–5 days. Etiologies include serum sickness (often induced by drugs such as penicillins, sulfas, salicylates, or barbiturates), connective-tissue disease (e.g., systemic lupus erythematosus or Sjögren’s syndrome), and infection (e.g., with hepatitis B virus, enteroviruses, or parasites). Malignancy, especially lymphoma, may be associated with fever and chronic urticaria (Chap. 72).
NODULAR ERUPTIONS
In immunocompromised hosts, nodular lesions often represent disseminated infection. Patients with disseminated candidiasis (often due to Candida tropicalis) may have a triad of fever, myalgias, and eruptive nodules (Chap. 240). Disseminated cryptococcosis lesions (Chap. 239) may resemble molluscum contagiosum (Chap. 220e). Necrosis of nodules should raise the suspicion of aspergillosis (Chap. 241) or mucormycosis (Chap. 242). Erythema nodosum presents with exquisitely tender nodules on the lower extremities. Sweet syndrome (Chap. 72) should be considered in individuals with multiple nodules and plaques, often so edematous that they give the appearance of vesicles or bullae. Sweet syndrome may occur in individuals with infection, inflammatory bowel disease, or malignancy and can also be induced by drugs.
PURPURIC ERUPTIONS
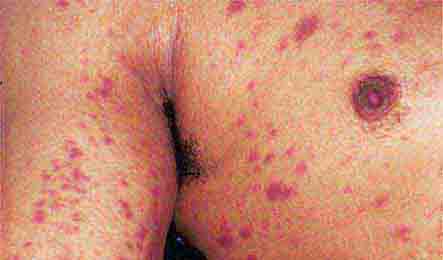
Acute meningococcemia (Chap. 180) classically presents in children as a petechial eruption, but initial lesions may appear as blanchable macules or urticaria. Rocky Mountain spotted fever should be considered in the differential diagnosis of acute meningococcemia. Echovirus 9 infection (Chap. 228) may mimic acute meningococcemia; patients should be treated as if they have bacterial sepsis because prompt differentiation of these conditions may be impossible. Large ecchymotic areas of purpura fulminans (Chaps. 180 and 325) reflect severe underlying disseminated intravascular coagulation, which may be due to infectious or noninfectious causes. The lesions of chronic meningococcemia (Chap. 180) may have a variety of morphologies, including petechial. Purpuric nodules may develop on the legs and resemble erythema nodosum but lack its exquisite tenderness. Lesions of disseminated gonococcemia (Chap. 181) are distinctive, sparse, countable hemorrhagic pustules, usually located near joints. The lesions of chronic meningococcemia and those of gonococcemia may be indistinguishable in terms of appearance and distribution. Viral hemorrhagic fever (Chaps. 233 and 234) should be considered in patients with an appropriate travel history and a petechial rash. Thrombotic thrombocytopenic purpura (Chaps. 72, 129, and 140) and hemolytic-uremic syndrome (Chaps. 140, 186, and 191) are closely related and are noninfectious causes of fever and petechiae. Cutaneous small-vessel vasculitis (leukocytoclastic vasculitis) typically manifests as palpable purpura and has a wide variety of causes (Chap. 72).
ERUPTIONS WITH ULCERS OR ESCHARS
The presence of an ulcer or eschar in the setting of a more widespread eruption can provide an important diagnostic clue. For example, the presence of an eschar may suggest the diagnosis of scrub typhus or rickettsialpox (Chap. 211) in the appropriate setting. In other illnesses (e.g., anthrax) (Chap. 261e), an ulcer or eschar may be the only skin manifestation.
25e | Atlas of Rashes Associated with Fever |
Given the extremely broad differential diagnosis, the presentation of a patient with fever and rash often poses a thorny diagnostic challenge for even the most astute and experienced clinician. Rapid narrowing of the differential by prompt recognition of a rash’s key features can result in appropriate and sometimes life-saving therapy. This atlas presents high-quality images of a variety of rashes that have an infectious etiology and are commonly associated with fever.
FIGURE 25e-1 A. Erythema leading to “slapped cheeks” appearance in erythema infectiosum (fifth disease) caused by parvovirus B19. B. Lacy reticular rash of erythema infectiosum. (Panel A reprinted from K Wolff, RA Johnson: Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology, 6th ed. New York, McGraw-Hill, 2009.)
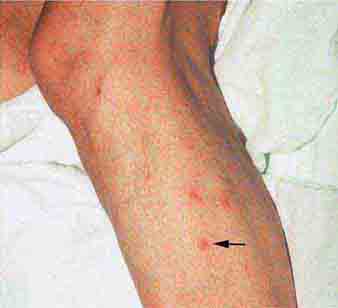
FIGURE 25e-2 Koplik’s spots, which manifest as white or bluish lesions with an erythematous halo on the buccal mucosa, usually occur in the first 2 days of measles symptoms and may briefly overlap the measles exanthem. The presence of the erythematous halo (arrow indicates one example) differentiates Koplik’s spots from Fordyce’s spots (ectopic sebaceous glands), which occur in the mouths of healthy individuals. (Courtesy of the Centers for Disease Control and Prevention.)
FIGURE 25e-3 In measles, discrete erythematous lesions become confluent on the face and neck over 2–3 days as the rash spreads downward to the trunk and arms, where lesions remain discrete. (Reprinted from K Wolff, RA Johnson: Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology, 5th ed. New York, McGraw-Hill, 2005.)
FIGURE 25e-4 In rubella, an erythematous exanthem spreads from the hairline downward and clears as it spreads. (Courtesy of Stephen E. Gellis, MD; with permission.)
FIGURE 25e-5 Exanthem subitum (roseola) occurs most commonly in young children. A diffuse maculopapular exanthem follows resolution of fever. (Courtesy of Stephen E. Gellis, MD; with permission.)
FIGURE 25e-6 Erythematous macules and papules are apparent on the trunk and arm of this patient with primary HIV infection. (Reprinted from K Wolff, RA Johnson: Color Atlas and Synopsis of Clinical Dermatology, 5th ed. New York, McGraw-Hill, 2005.)
FIGURE 25e-7 This exanthematous, drug-induced eruption consists of brightly erythematous macules and papules, some of which are confluent, distributed symmetrically on the trunk and extremities. Ampicillin caused this rash. (Reprinted from K Wolff, RA Johnson: Color Atlas and Synopsis of Clinical Dermatology, 5th ed. New York, McGraw-Hill, 2005.)
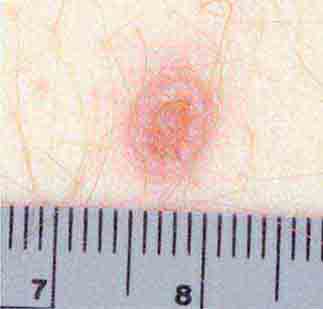
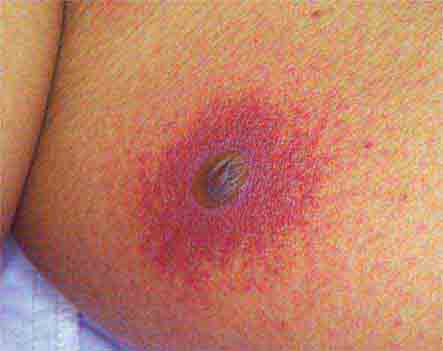
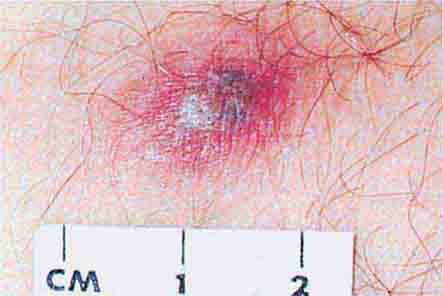
FIGURE 25e-8 Erythema migrans is the early cutaneous manifestation of Lyme disease and is characterized by erythematous annular patches, often with a central erythematous focus at the tick-bite site. (Reprinted from RP Usatine et al: Color Atlas of Family Medicine, 2nd ed. New York, McGraw-Hill, 2013. Courtesy of Thomas Corson, MD.)
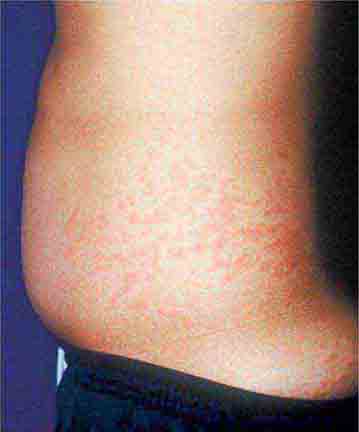
FIGURE 25e-9 Rose spots are evident as erythematous macules on the trunk of this patient with typhoid fever. (Courtesy of the Centers for Disease Control and Prevention.)
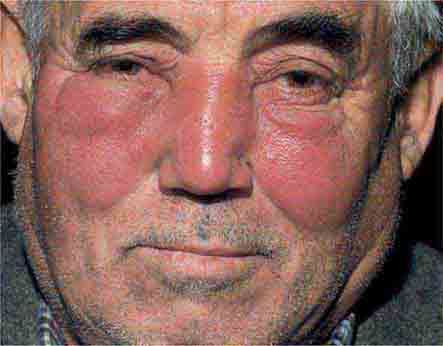
FIGURE 25e-10 Systemic lupus erythematosus showing prominent malar erythema and minimal scaling. Involvement of other sun-exposed sites is also common. (Reprinted from K Wolff, RA Johnson: Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology, 6th ed. New York, McGraw-Hill, 2009.)
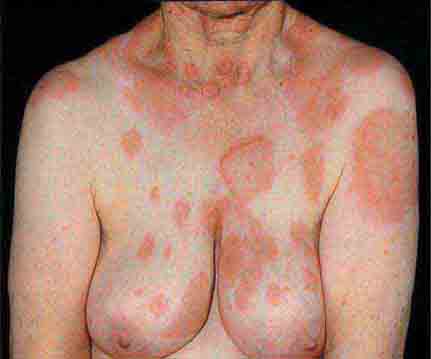
FIGURE 25e-11 Subacute lupus erythematosus on the upper chest, with brightly erythematous and slightly edematous coalescent papules and plaques. (Reprinted from K Wolff, RA Johnson: Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology, 6th ed. New York, McGraw-Hill, 2009.)
FIGURE 25e-12 Chronic discoid lupus erythematosus. Violaceous, hyperpigmented, atrophic plaques, often with evidence of follicular plugging (which may result in scarring), are characteristic of this cutaneous form of lupus. (Reprinted from K Wolff, RA Johnson, AP Saavedra: Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology, 7th ed. New York, McGraw-Hill, 2013.)
FIGURE 25e-13 The rash of Still’s disease typically exhibits evanescent, erythematous papules that appear at the height of fever on the trunk and proximal extremities. (Courtesy of Stephen E. Gellis, MD; with permission.)
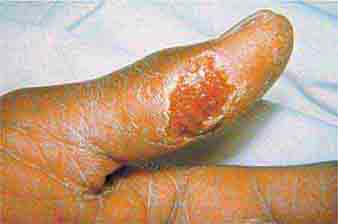
FIGURE 25e-14 Impetigo is a superficial group A streptococcal or Staphylococcus aureus infection consisting of honey-colored crusts and erythematous weeping erosions. (Reprinted from K Wolff, RA Johnson: Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology, 6th ed. New York, McGraw-Hill, 2009.)
FIGURE 25e-15 Erysipelas is a group A streptococcal infection of the superficial dermis and consists of well-demarcated, erythematous, edematous, warm plaques. (Reprinted from K Wolff, RA Johnson, AP Saavedra: Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology, 7th ed. New York, McGraw-Hill, 2013.)
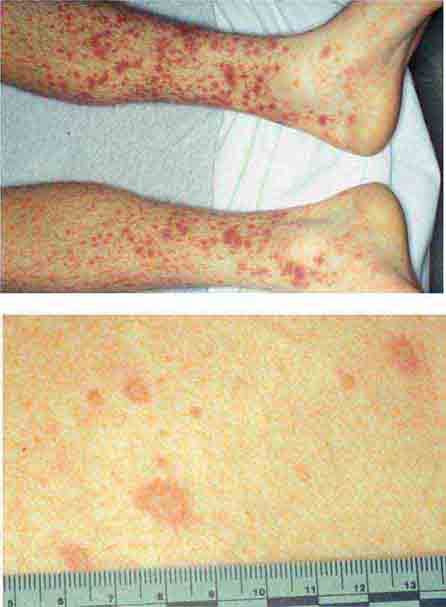
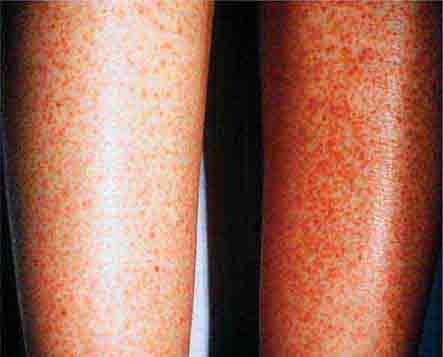
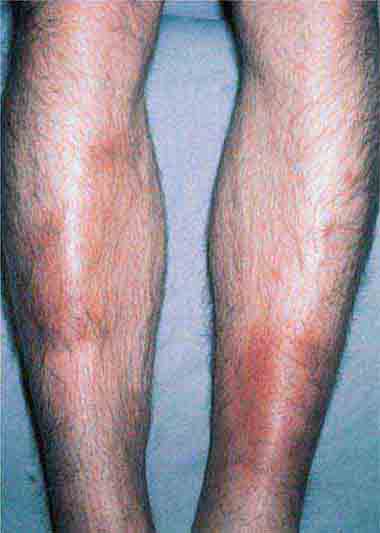
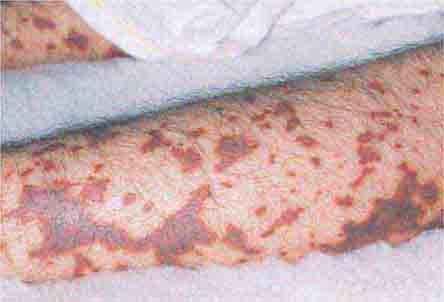
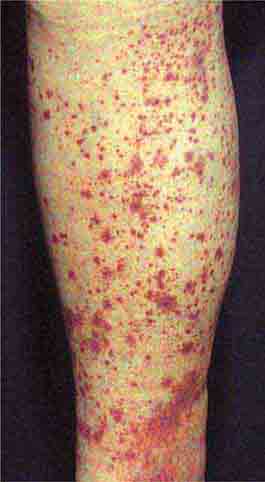
FIGURE 25e-16 Top: Petechial lesions of Rocky Mountain spotted fever on the lower legs and soles of a young, otherwise healthy patient. Bottom: Close-up of lesions from the same patient. (Courtesy of Lindsey Baden, MD; with permission.)
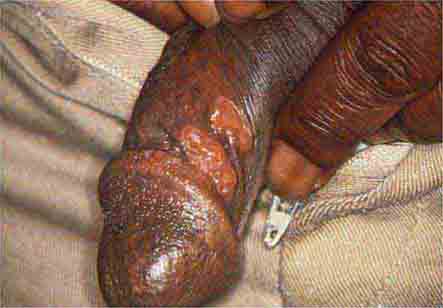
FIGURE 25e-17 Primary syphilis with firm, nontender chancres. (Courtesy of M. Rein and the Centers for Disease Control and Prevention.)
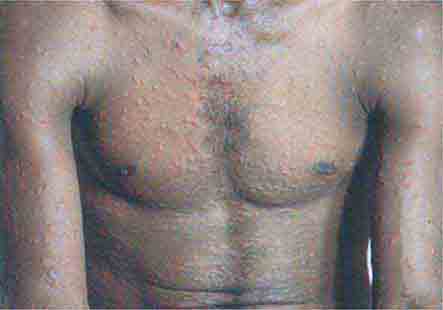
FIGURE 25e-18 Secondary syphilis, demonstrating the papulosquamous truncal eruption.
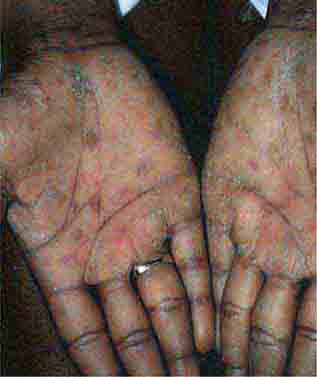
FIGURE 25e-19 Secondary syphilis commonly affects the palms and soles with scaling, firm, red-brown papules.
FIGURE 25e-20 Condylomata lata are moist, somewhat verrucous intertriginous plaques seen in secondary syphilis.
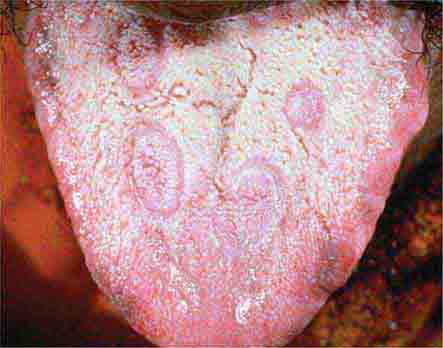
FIGURE 25e-21 Mucous patches on the tongue of a patient with secondary syphilis. (Courtesy of Ron Roddy; with permission.)
FIGURE 25e-22 Petechial lesions in a patient with atypical measles. (Courtesy of Stephen E. Gellis, MD; with permission.)
FIGURE 25e-23 Tender vesicles and erosions in the mouth of a patient with hand-foot-and-mouth disease. (Courtesy of Stephen E. Gellis, MD; with permission.)
FIGURE 25e-24 Septic emboli with hemorrhage and infarction due to acute Staphylococcus aureus endocarditis. (Courtesy of Lindsey Baden, MD; with permission.)
FIGURE 25e-25 Erythema multiforme is characterized by erythematous plaques with a target or iris morphology, sometimes with a vesicle in the center. It usually represents a hypersensitivity reaction to infections (especially herpes simplex virus or Mycoplasma pneumoniae) or drugs. (Reprinted from K Wolff, RA Johnson: Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology, 6th ed. New York, McGraw-Hill, 2009.)
FIGURE 25e-26 Scarlet fever exanthem. Finely punctuated erythema has become confluent (scarlatiniform); accentuation of linear erythema in body folds (Pastia’s lines) is seen here. (Reprinted from K Wolff, RA Johnson: Color Atlas and Synopsis of Clinical Dermatology, 6th ed. New York, McGraw-Hill, 2009.)
FIGURE 25e-27 Erythema progressing to bullae with resulting sloughing of the entire thickness of the epidermis occurs in toxic epidermal necrolysis. This reaction was due to a sulfonamide. (Reprinted from K Wolff, RA Johnson: Color Atlas and Synopsis of Clinical Dermatology, 5th ed. New York, McGraw-Hill, 2005.)
FIGURE 25e-28 Diffuse erythema and scaling are present in this patient with psoriasis and the exfoliative erythroderma syndrome. (Reprinted from K Wolff, RA Johnson: Color Atlas and Synopsis of Clinical Dermatology, 6th ed. New York, McGraw-Hill, 2009.)
FIGURE 25e-29 This infant with staphylococcal scalded skin syndrome demonstrates generalized desquamation. (Reprinted from K Wolff, RA Johnson: Color Atlas and Synopsis of Clinical Dermatology, 6th ed. New York, McGraw-Hill, 2009.)
FIGURE 25e-30 Fissuring of the lips and an erythematous exanthem are evident in this patient with Kawasaki disease. (Courtesy of Stephen E. Gellis, MD; with permission.)
FIGURE 25e-31 Numerous varicella lesions at various stages of evolution: vesicles on an erythematous base and umbilicated vesicles, which then develop into crusting lesions. (Courtesy of the Centers for Disease Control and Prevention.)
FIGURE 25e-32 Lesions of disseminated zoster at different stages of evolution, including pustules and crusting, similar to varicella. Note nongrouping of lesions, in contrast to herpes simplex or zoster. (Reprinted from K Wolff, RA Johnson, AP Saavedra: Color Atlas and Synopsis of Clinical Dermatology, 7th ed. New York, McGraw-Hill, 2013.)
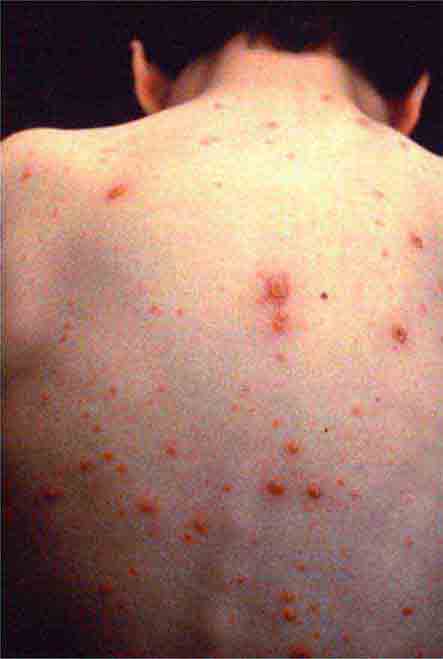
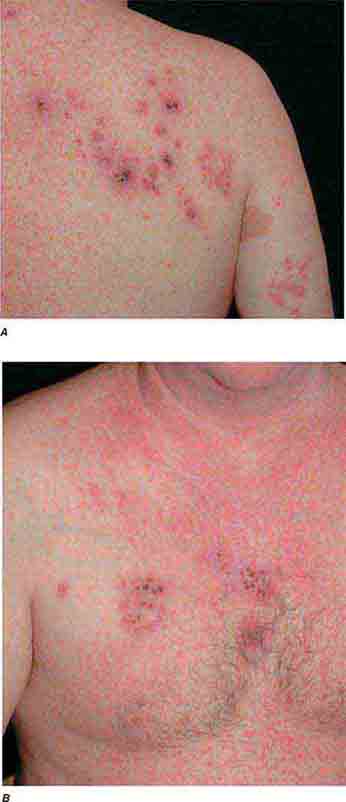
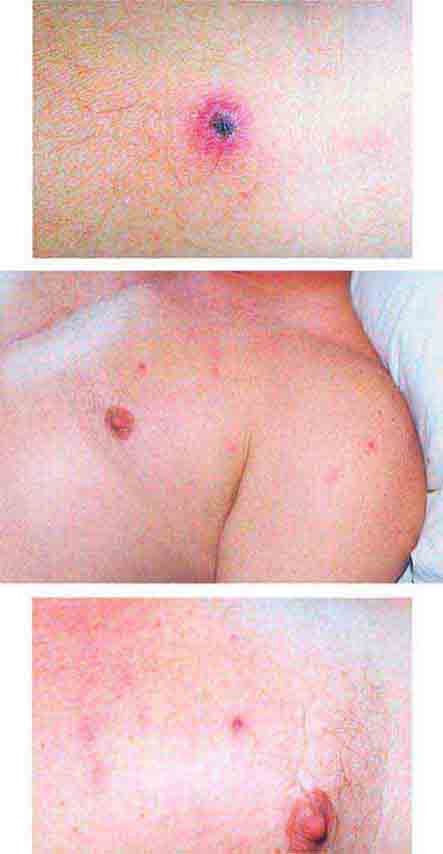
FIGURE 25e-33 Herpes zoster is seen in this patient taking prednisone. Grouped vesicles and crusted lesions are seen in the T2 dermatome on the back and arm (A) and on the right side of the chest (B). (Reprinted from K Wolff, RA Johnson: Color Atlas and Synopsis of Clinical Dermatology, 6th ed. New York, McGraw-Hill, 2009.)
FIGURE 25e-34 Top: Eschar at the site of the mite bite in a patient with rickettsialpox. Middle: Papulovesicular lesions on the trunk of the same patient. Bottom: Close-up of lesions from the same patient. (Reprinted from A Krusell et al: Emerg Infect Dis 8:727, 2002.)
FIGURE 25e-35 Ecthyma gangrenosum in a neutropenic patient with Pseudomonas aeruginosa bacteremia.
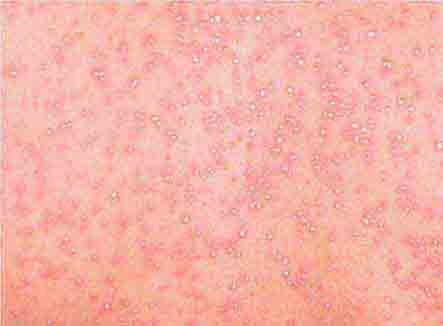
FIGURE 25e-36 Urticaria showing characteristic discrete and confluent, edematous, erythematous papules and plaques. (Reprinted from K Wolff, RA Johnson, AP Saavedra: Color Atlas and Synopsis of Clinical Dermatology, 7th ed. New York, McGraw-Hill, 2013.)
FIGURE 25e-37 Disseminated cryptococcal infection. A liver transplant recipient developed six cutaneous lesions similar to the one shown. Biopsy and serum antigen testing demonstrated Cryptococcus. Important features of the lesion include a benign-appearing fleshy papule with central umbilication resembling molluscum contagiosum. (Courtesy of Lindsey Baden, MD; with permission.)
FIGURE 25e-38 Disseminated candidiasis. Tender, erythematous, nodular lesions developed in a neutropenic patient with leukemia who was undergoing induction chemotherapy. (Courtesy of Lindsey Baden, MD; with permission.)
FIGURE 25e-39 Disseminated Aspergillus infection. Multiple necrotic lesions developed in this neutropenic patient undergoing hematopoietic stem cell transplantation. The lesion in the photograph is on the inner thigh and is several centimeters in diameter. Biopsy demonstrated infarction caused by Aspergillus fumigatus. (Courtesy of Lindsey Baden, MD; with permission.)
FIGURE 25e-40 Erythema nodosum is a panniculitis characterized by tender, deep-seated nodules and plaques usually located on the lower extremities. (Courtesy of Robert Swerlick, MD; with permission.)
FIGURE 25e-41 Sweet syndrome is an erythematous indurated plaque with a pseudovesicular border. (Courtesy of Robert Swerlick, MD; with permission.)
FIGURE 25e-42 Fulminant meningococcemia with extensive angular purpuric patches. (Courtesy of Stephen E. Gellis, MD; with permission.)
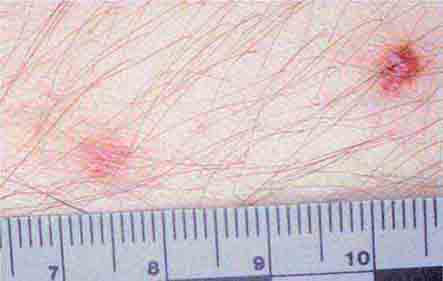
FIGURE 25e-43 Erythematous papular lesions are seen on the leg of this patient with chronic meningococcemia (arrow indicates a lesion).
FIGURE 25e-44 Disseminated gonococcemia in the skin is seen as hemorrhagic papules and pustules with purpuric centers in a centrifugal distribution. (Courtesy of Daniel M. Musher, MD; with permission.)
FIGURE 25e-45 Palpable purpuric papules on the lower leg are seen in this patient with cutaneous small-vessel hypersensitivity vasculitis. (Reprinted from K Wolff, RA Johnson: Color Atlas and Synopsis of Clinical Dermatology, 6th ed. New York, McGraw-Hill, 2009.)
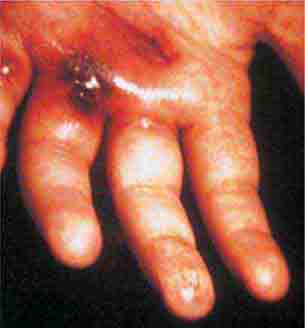
FIGURE 25e-46 The thumb of a patient with a necrotic ulcer of tularemia. (Courtesy of the Centers for Disease Control and Prevention.)
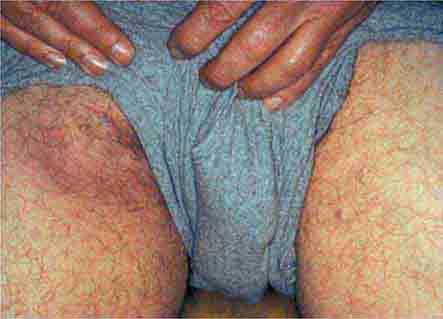
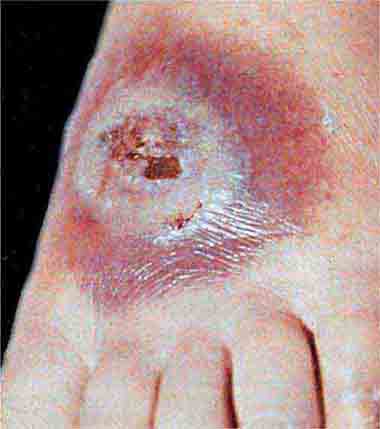
FIGURE 25e-47 This 50-year-old man developed high fever and massive inguinal lymphadenopathy after a small ulcer healed on his foot. Tularemia was diagnosed. (Courtesy of Lindsey Baden, MD; with permission.)
FIGURE 25e-48 This painful trypanosomal chancre developed at the site of a tsetse fly bite on the dorsum of the foot. Trypanosoma brucei was diagnosed from an aspirate of the ulcer. (Courtesy of Edward T. Ryan, MD. N Engl J Med 346:2069, 2002; with permission.)
FIGURE 25e-49 Drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS). This patient developed a progressive eruption exhibiting early desquamation after taking phenobarbital. There was also associated lymphadenopathy and hepatomegaly. (Courtesy of Peter Lio, MD; with permission.)
FIGURE 25e-50 Many small, nonfollicular pustules are seen against a background of erythema in this patient with acute generalized eruptive pustulosis (AGEP). The rash began in body folds and progressed to cover the trunk and face. (Reprinted from K Wolff, RA Johnson: Color Atlas and Synopsis of Clinical Dermatology, 6th ed. New York, McGraw-Hill, 2009.)
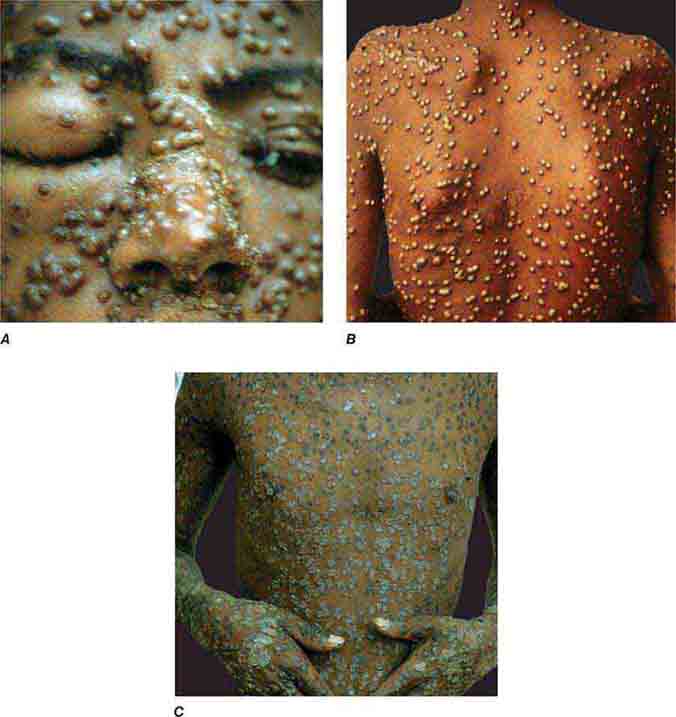
FIGURE 25e-51 Smallpox is shown with many pustules on the face, becoming confluent (A), and on the trunk (B). Pustules are all in the same stage of development. C. Crusting, healing lesions are noted on the trunk, arms, and hands. (Reprinted from K Wolff, RA Johnson: Color Atlas and Synopsis of Clinical Dermatology, 6th ed. New York, McGraw-Hill, 2009.)
26 | Fever of Unknown Origin |
DEFINITION
Clinicians commonly refer to any febrile illness without an initially obvious etiology as fever of unknown origin (FUO). Most febrile illnesses either resolve before a diagnosis can be made or develop distinguishing characteristics that lead to a diagnosis. The term FUO should be reserved for prolonged febrile illnesses without an established etiology despite intensive evaluation and diagnostic testing. This chapter focuses on classic FUO in the adult patient.
FUO was originally defined by Petersdorf and Beeson in 1961 as an illness of >3 weeks’ duration with fever of ≥38.3°C (101°F) on two occasions and an uncertain diagnosis despite 1 week of inpatient evaluation. Nowadays, most patients with FUO are hospitalized if their clinical condition requires it, but not for diagnostic purposes only; thus the in-hospital evaluation requirement has been eliminated from the definition. The definition of FUO has been further modified by the exclusion of immunocompromised patients, whose workup requires an entirely different diagnostic and therapeutic approach. For the optimal comparison of patients with FUO in different geographic areas, it has been proposed that the quantitative criterion (diagnosis uncertain after 1 week of evaluation) be changed to a qualitative criterion that requires the performance of a specific list of investigations. Accordingly, FUO is now defined as:
1. Fever >38.3°C (101°F) on at least two occasions
2. Illness duration of ≥3 weeks
3. No known immunocompromised state
4. Diagnosis that remains uncertain after a thorough history-taking, physical examination, and the following obligatory investigations: determination of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level; platelet count; leukocyte count and differential; measurement of levels of hemoglobin, electrolytes, creatinine, total protein, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, creatine kinase, ferritin, antinuclear antibodies, and rheumatoid factor; protein electrophoresis; urinalysis; blood cultures (n = 3); urine culture; chest x-ray; abdominal ultrasonography; and tuberculin skin test (TST).
ETIOLOGY AND EPIDEMIOLOGY
The range of FUO etiologies has evolved over time as a result of changes in the spectrum of diseases causing FUO, the widespread use of antibiotics, and the availability of new diagnostic techniques. The proportion of cases caused by intraabdominal abscesses and tumors, for example, has decreased because of earlier detection by CT and ultrasound. In addition, infective endocarditis is a less frequent cause because blood culture and echocardiographic techniques have improved. Conversely, some diagnoses, such as acute HIV infection, were unknown four decades ago.
![]() Table 26-1 summarizes the findings of several large studies on FUO conducted over the past 20 years. In general, infection accounts for about 20–25% of cases of FUO in Western countries; next in frequency are neoplasms and noninfectious inflammatory diseases (NIIDs), the latter including “collagen or rheumatic diseases,” vasculitis syndromes, and granulomatous disorders. In geographic areas outside the West, infections are a much more common cause of FUO (43% vs 22%), while the proportions of cases due to NIIDs and neoplasms are similar. Up to 50% of cases caused by infections in patients with FUO outside Western nations are due to tuberculosis, which is a less common cause in the United States and Western Europe. The number of FUO patients diagnosed with NIIDs probably will not decrease in the near future, as fever may precede more typical manifestations or serologic evidence by months in these diseases. Moreover, many NIIDs can be diagnosed only after prolonged observation and exclusion of other diseases.
Table 26-1 summarizes the findings of several large studies on FUO conducted over the past 20 years. In general, infection accounts for about 20–25% of cases of FUO in Western countries; next in frequency are neoplasms and noninfectious inflammatory diseases (NIIDs), the latter including “collagen or rheumatic diseases,” vasculitis syndromes, and granulomatous disorders. In geographic areas outside the West, infections are a much more common cause of FUO (43% vs 22%), while the proportions of cases due to NIIDs and neoplasms are similar. Up to 50% of cases caused by infections in patients with FUO outside Western nations are due to tuberculosis, which is a less common cause in the United States and Western Europe. The number of FUO patients diagnosed with NIIDs probably will not decrease in the near future, as fever may precede more typical manifestations or serologic evidence by months in these diseases. Moreover, many NIIDs can be diagnosed only after prolonged observation and exclusion of other diseases.
ETIOLOGY OF FEVER OF UNKNOWN ORIGIN (FUO) OVER THE PAST 20 YEARS: FINDINGS FROM LARGE FUO STUDIES |
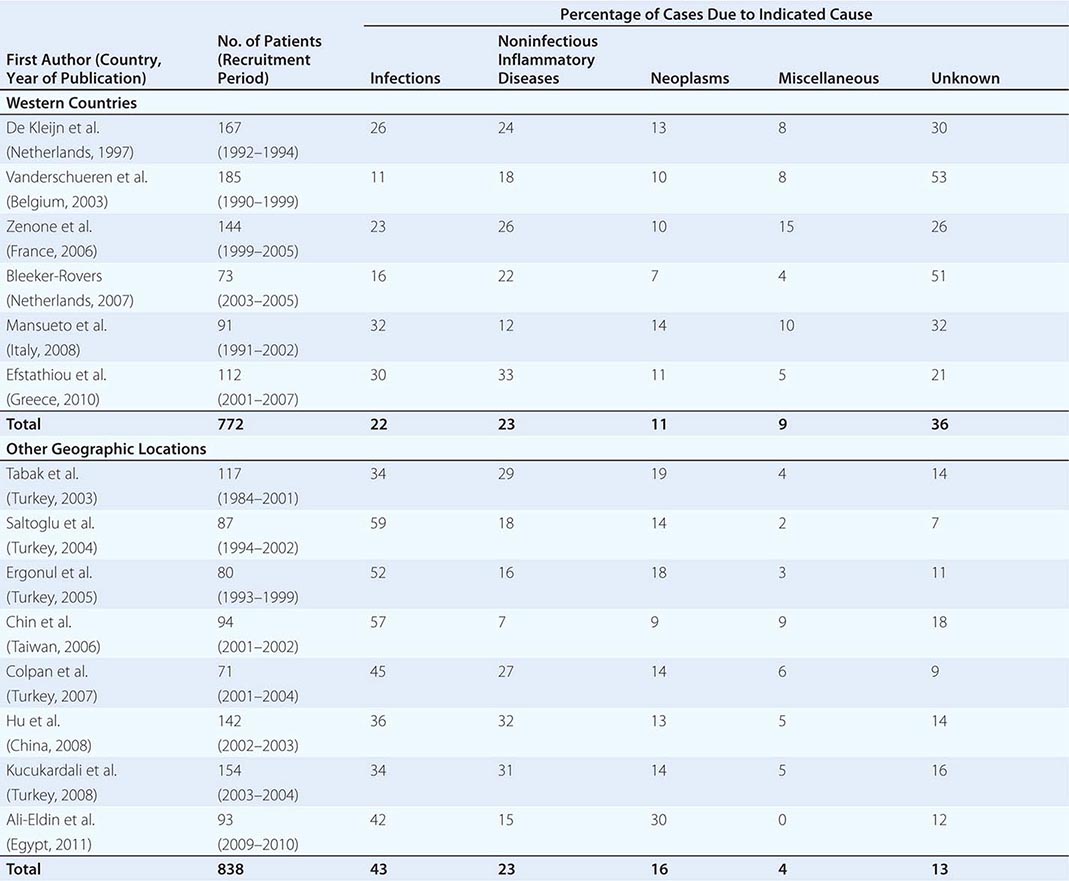
In the West, the percentage of undiagnosed cases of FUO has increased in more recent studies. An important factor contributing to the seemingly high diagnostic failure rate is that a diagnosis is more often being established before 3 weeks have elapsed, given that patients with fever tend to seek medical advice earlier and better diagnostic techniques, such as CT and MRI, are widely available; therefore, only the cases that are more difficult to diagnose continue to meet the criteria for FUO. Furthermore, most patients who have FUO without a diagnosis currently do well, and thus a less aggressive diagnostic approach may be used in clinically stable patients once diseases with immediate therapeutic or prognostic consequences have been ruled out to a reasonable extent. This factor may be especially relevant to patients with recurrent fever who are asymptomatic in between febrile episodes. In patients with recurrent fever (defined as repeated episodes of fever interspersed with fever-free intervals of at least 2 weeks and apparent remission of the underlying disease), the chance of attaining an etiologic diagnosis is <50%.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis for FUO is extensive, but it is important to remember that FUO is far more often caused by an atypical presentation of a rather common disease than by a very rare disease. Table 26-2 presents an overview of possible causes of FUO. An atypical presentation of endocarditis, diverticulitis, vertebral osteomyelitis, and extrapulmonary tuberculosis are the more common infectious disease diagnoses. Q fever and Whipple’s disease are quite rare but should always be kept in mind as a cause of FUO since the presenting symptoms can be nonspecific. Serologic testing for Q fever, which results from exposure to animals or animal products, should be performed when the patient lives in a rural area or has a history of heart valve disease, an aortic aneurysm, or a vascular prosthesis. In patients with unexplained symptoms localized to the central nervous system (CNS), gastrointestinal tract, or joints, polymerase chain reaction (PCR) testing for Tropheryma whipplei should be performed. Travel to or (former) residence in tropical countries or the American Southwest should lead to consideration of infectious diseases such as malaria, leishmaniasis, histoplasmosis, or coccidioidomycosis. Fever with signs of endocarditis and negative blood culture results poses a special problem. Culture-negative endocarditis may be due to difficult-to-culture bacteria such as nutritionally variant bacteria, HACEK organisms (Haemophilus parainfluenzae, H. paraphrophilus, Aggregatibacter species [actinomycetemcomitans, aphrophilus], Cardiobacterium species [hominis, valvarum], Eikenella corrodens, and Kingella kingae; discussed below), Coxiella burnetii (as indicated above), T. whipplei, and Bartonella species. Marantic endocarditis is a sterile thrombotic disease that occurs as a paraneoplastic phenomenon, especially with adenocarcinomas. Sterile endocarditis is also seen in the context of systemic lupus erythematosus and antiphospholipid syndrome.
ALL REPORTED CAUSES OF FUOa |
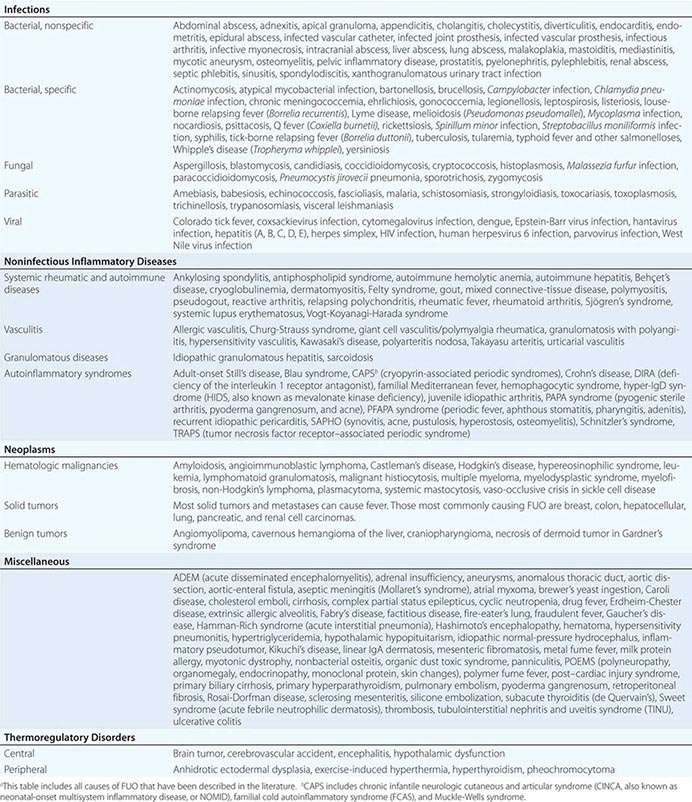
Of the NIIDs, large-vessel vasculitis, polymyalgia rheumatica, sarcoidosis, familial Mediterranean fever, and adult-onset Still’s disease are rather common diagnoses in patients with FUO. The hereditary autoinflammatory syndromes are very rare and usually present in young patients. Schnitzler’s syndrome, which can present at any age, is uncommon but can often be diagnosed easily in a patient with FUO who presents with urticaria, bone pain, and monoclonal gammopathy.
Although most tumors can present with fever, malignant lymphoma is by far the most common diagnosis of FUO among the neoplasms. Sometimes the fever even precedes lymphadenopathy detectable by physical examination.
Apart from drug-induced fever and exercise-induced hyperthermia, none of the miscellaneous causes of fever is found very frequently in patients with FUO. Virtually all drugs can cause fever, even that commencing after long-term use. Drug-induced fever, including DRESS (drug reaction with eosinophilia and systemic symptoms; Fig. 25e-49), is often accompanied by eosinophilia and also by lymphadenopathy, which can be extensive. More common causes of drug-induced fever are allopurinol, carbamazepine, lamotrigine, phenytoin, sulfasalazine, furosemide, antimicrobial drugs (especially sulfonamides, minocycline, vancomycin, β-lactam antibiotics, and isoniazid), some cardiovascular drugs (e.g., quinidine), and some antiretroviral drugs (e.g., nevirapine). Exercise-induced hyperthermia (Chap. 479e) is characterized by an elevated body temperature that is associated with moderate to strenuous exercise lasting from half an hour up to several hours without an increase in CRP level or ESR; typically these patients sweat during the temperature elevation. Factitious fever (fever artificially induced by the patient—for example, by IV injection of contaminated water) should be considered in all patients but is more common among young women in health care professions. In fraudulent fever, the patient is normothermic but manipulates the thermometer. Simultaneous measurements at different body sites (rectum, ear, mouth) should rapidly identify this diagnosis. Another clue to fraudulent fever is a dissociation between pulse rate and temperature.
Previous studies of FUO have shown that a diagnosis is more likely in elderly patients than in younger age groups. In many cases, FUO in the elderly results from an atypical manifestation of a common disease, among which giant cell arteritis and polymyalgia rheumatica are most frequently involved. Tuberculosis is the most common infectious disease associated with FUO in elderly patients, occurring much more often than in younger patients. As many of these diseases are treatable, it is well worth pursuing the cause of fever in elderly patients.
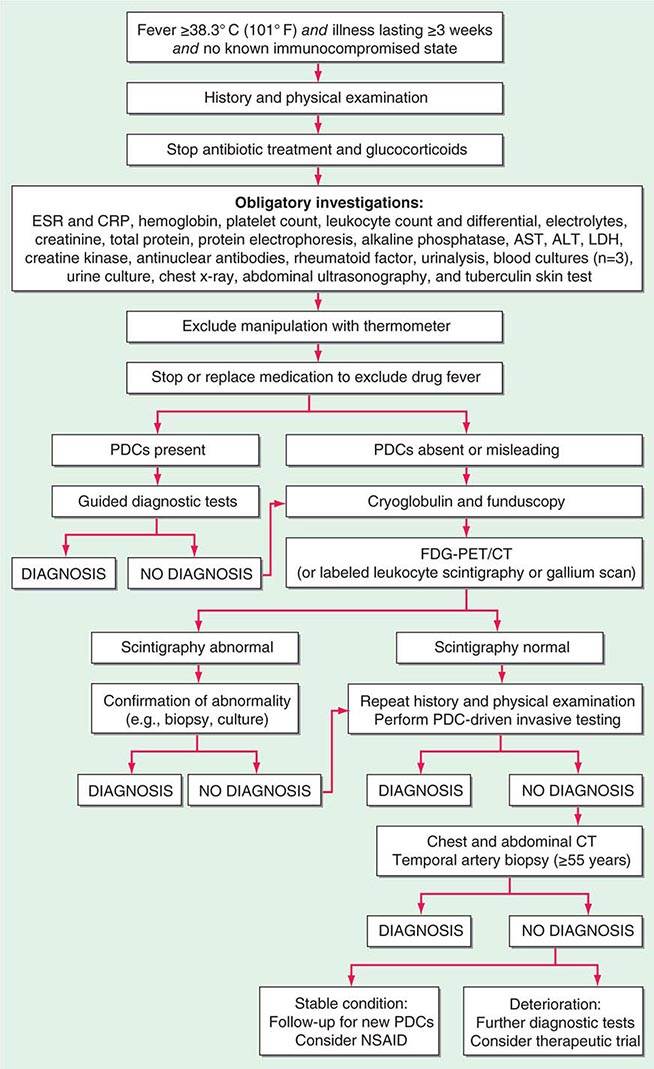
FIGURE 26-1 Structured approach to patients with FUO. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography combined with low-dose computed tomography; LDH, lactate dehydrogenase; PDCs, potentially diagnostic clues (all localizing signs, symptoms, and abnormalities potentially pointing toward a diagnosis); NSAID, nonsteroidal anti-inflammatory drug.
ALL REPORTED CAUSES OF RECURRENT FEVERa |