High-Yield Terms to Learn
Bicarbonate diuretic A diuretic that selectively increases sodium bicarbonate excretion. Example: a carbonic anhydrase inhibitor Diluting segment A segment of the nephron that removes solute without water; the thick ascending limb and the distal convoluted tubule are active salt-absorbing segments that are not permeable by water Hyperchloremic metabolic acidosis A shift in body electrolyte and pH balance involving elevated chloride, diminished bicarbonate concentration, and a decrease in pH in the blood. Typical result of bicarbonate diuresis Hypokalemic metabolic alkalosis A shift in body electrolyte balance and pH involving a decrease in serum potassium and an increase in blood pH. Typical result of loop and thiazide diuretic actions Nephrogenic diabetes insipidus Loss of urine-concentrating ability in the kidney caused by lack of responsiveness to antidiuretic hormone (ADH is normal or high) Pituitary diabetes insipidus Loss of urine-concentrating ability in the kidney caused by lack of antidiuretic hormone (ADH is low or absent) Potassium-sparing diuretic A diuretic that reduces the exchange of potassium for sodium in the collecting tubule; a drug that increases sodium and reduces potassium excretion. Example: aldosterone antagonists. Uricosuric diuretic A diuretic that increases uric acid excretion, usually by inhibiting uric acid reabsorption in the proximal tubule. Example: ethacrynic acid
Renal Transport Mechanisms & Diuretic Drug Groups
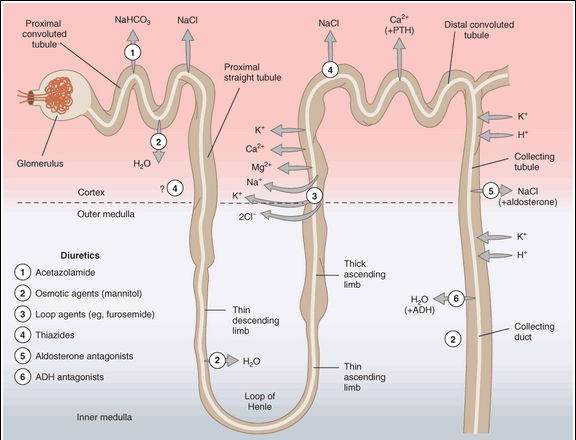
The kidney filters both inorganic and organic solutes at the glomerulus and must recover a significant percentage of these substances before excretion in the urine. The major transport mechanisms for the recovery of ions and water in the various segments of the nephron are shown in Figure 15-1. Because the mechanisms for reabsorption of salt and water differ in each of the 4 major tubular segments, the diuretics acting in these segments each have differing mechanisms of action. Most diuretics act from the luminal side of the membrane and must be present in the urine. They are filtered at the glomerulus, and some are also secreted by the weak acid-secretory carrier in the proximal tubule. An exception is the aldosterone receptor antagonist group (eg, spironolactone and eplerenone), drugs that enter the collecting tubule cell from the basolateral side and bind to the cytoplasmic aldosterone receptor.
FIGURE 15-1
Tubule transport systems in the kidney and sites of action of diuretics. Circles with arrows denote known ion cotransporters that are targets of the diuretics indicated by the numerals. Question marks denote preliminary or incompletely documented suggestions for the location of certain drug effects.
(Modified and reproduced, with permission, from Katzung BG, editor: Basic & Clinical Pharmacology, 11th ed. McGraw-Hill, 2009: Fig. 15-1.)
Proximal Convoluted Tubule (PCT)
This segment carries out isosmotic reabsorption of amino acids, glucose, and numerous cations. It is also the major site for sodium chloride and sodium bicarbonate reabsorption. The proximal tubule is responsible for 60-70% of the total reabsorption of sodium. No currently available drug directly acts on NaCl reabsorption in the PCT. The mechanism for bicarbonate reabsorption is shown in Figure 15-2. Bicarbonate itself is poorly reabsorbed through the luminal membrane, but conversion of bicarbonate to carbon dioxide via carbonic acid permits rapid reabsorption of the carbon dioxide. Bicarbonate can then be regenerated from carbon dioxide within the tubular cell and transported into the interstitium. Sodium is separately reabsorbed from the lumen in exchange for hydrogen ions and transported into the interstitial space by the sodium-potassium pump (Na+/K+ ATPase). Carbonic anhydrase, the enzyme required for the bicarbonate reabsorption process on the brush border and in the cytoplasm, is the target of carbonic anhydrase inhibitor diuretic drugs. Active secretion and reabsorption of weak acids and bases also occurs in the proximal tubule. Most weak acid transport occurs in the straight S2 segment, distal to the convoluted part. Uric acid transport is especially important and is targeted by some of the drugs used in treating gout (Chapter 36). Weak bases are transported in the S1 and S2 segments.
FIGURE 15-2
Mechanism of sodium bicarbonate reabsorption in the proximal tubule cell. NHE3, Na+/H+ exchanger 3; CA, carbonic anhydrase.
(Reproduced, with permission, from Katzung BG, editor: Basic & Clinical Pharmacology, 11th ed. McGraw-Hill, 2009: Fig. 15-2.)
Carbonic Anhydrase Inhibitors
Prototypes and Mechanism of Action
Acetazolamide is the prototypic agent. These diuretics are sulfonamide derivatives. The mechanism of action is inhibition of carbonic anhydrase in the brush border and cytoplasm (Figure 15-2). Carbonic anhydrase is also found in other tissues and plays an important role in the secretion of cerebrospinal fluid and aqueous humor. Acetazolamide inhibits carbonic anhydrase in all tissues of the body.
Effects
The major renal effect is bicarbonate diuresis (ie, sodium bicarbonate is excreted); body bicarbonate is depleted, and metabolic acidosis results. As increased sodium is presented to the cortical collecting tubule, some of the excess sodium is reabsorbed and potassium is secreted, resulting in significant potassium wasting (Table 15-1). As a result of bicarbonate depletion, sodium bicarbonate excretion slows—even with continued diuretic administration—and the diuresis is self-limiting within 2-3 days. Secretion of bicarbonate into aqueous humor by the ciliary epithelium in the eye and into the cerebrospinal fluid by the choroid plexus is reduced. In the eye, a useful reduction in intraocular pressure can be achieved. In the central nervous system (CNS), acidosis of the cerebrospinal fluid results in hyperventilation, which can protect against high-altitude sickness. The ocular and cerebrospinal fluid effects are not self-limiting.
TABLE 15-1 Electrolyte changes produced by diuretic drugs.
Amount in Urine Group NaCl NaHCO3
K+
Body pH Carbonic anhydrase inhibitors 



 Acidosis Loop diuretics
Acidosis Loop diuretics 


 —
—  Alkalosis Thiazides
Alkalosis Thiazides 

 ,—
,—  Alkalosis K+-sparing diuretics
Alkalosis K+-sparing diuretics
 —
—  Acidosis
Acidosis
Clinical Uses
A major application of carbonic anhydrase inhibitors is in the treatment of severe acute glaucoma (see Table 10-3). Acetazolamide must be administered orally or parenterally, but topical analogs are now available (dorzolamide, brinzolamide) for chronic use in the eye. Carbonic anhydrase inhibitors are also used to prevent acute mountain (high-altitude) sickness. These agents are used for their diuretic effect only if edema is accompanied by significant metabolic alkalosis.
Toxicity
Drowsiness and paresthesias are commonly reported after oral therapy. Cross-allergenicity between these and all other sulfonamide derivatives (other sulfonamide diuretics, hypoglycemic agents, antibacterial sulfonamides) is uncommon but does occur. Alkalinization of the urine by these drugs may cause precipitation of calcium salts and formation of renal stones. Renal potassium wasting may be marked. Patients with hepatic impairment often excrete large amounts of ammonia in the urine in the form of ammonium ion. If they are given acetazolamide, alkalinization of the urine prevents conversion of ammonia to ammonium ion. As a result, they may develop hepatic encephalopathy because of increased ammonia reabsorption and hyperammonemia.
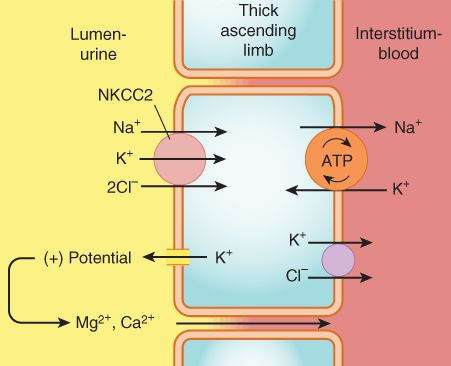
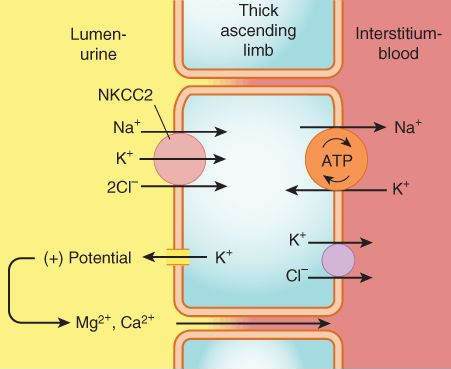
Thick Ascending Limb of the Loop of Henle (TAL)
This segment pumps sodium, potassium, and chloride out of the lumen into the interstitium of the kidney. It is also a major site of calcium and magnesium reabsorption, as shown in Figure 15-3. Reabsorption of sodium, potassium, and chloride are all accomplished by a Na+/K+/2Cl- carrier, which is the target of the loop diuretics. This cotransporter provides part of the concentration gradient for the countercurrent concentrating mechanism in the kidney and is responsible for the reabsorption of 20-30% of the sodium filtered at the glomerulus. Because potassium is pumped into the cell from both the luminal and basal sides, an escape route must be provided; this occurs into the lumen via a potassium-selective channel. Because the potassium diffusing through these channels is not accompanied by an anion, a net positive charge is set up in the lumen. This positive potential drives the reabsorption of calcium and magnesium.
FIGURE 15-3
Mechanism of sodium, potassium, and chloride reabsorption by the transporter NKCC2 in the thick ascending limb of the loop of Henle. Note that pumping of potassium into the cell from both the lumen and the interstitium would result in unphysiologically high intracellular K+ concentration. This is avoided by movement of K+ down its concentration gradient back into the lumen, carrying with it excess positive charge. This positive charge drives the reabsorption of calcium and magnesium.
(Reproduced, with permission, from Katzung BG, editor: Basic & Clinical Pharmacology, 11th ed. McGraw-Hill, 2009: Fig. 15-3.)
Loop Diuretics
Prototypes and Mechanism of Action
Furosemide is the prototypical loop agent. Furosemide, bumetanide, and torsemide are sulfonamide derivatives. Ethacrynic acid
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree