Disorders of Sodium and Water Homeostasis
KEY CONCEPTS
![]() Blood volume and plasma osmolality are tightly regulated in the human body because they are essential for normal cellular function. Water balance determines the serum sodium concentration, and sodium balance determines the water status.
Blood volume and plasma osmolality are tightly regulated in the human body because they are essential for normal cellular function. Water balance determines the serum sodium concentration, and sodium balance determines the water status.
![]() Hypovolemic hypotonic hyponatremia is relatively common in patients taking thiazide diuretics; however, thiazide-induced hyponatremia is usually mild and relatively asymptomatic.
Hypovolemic hypotonic hyponatremia is relatively common in patients taking thiazide diuretics; however, thiazide-induced hyponatremia is usually mild and relatively asymptomatic.
![]() Euvolemic (isovolemic) hyponatremia is most often caused by the syndrome of inappropriate secretion of antidiuretic hormone (SIADH). Common causes of SIADH include some cancers, central nervous system (CNS) and pulmonary disorders, and certain drugs.
Euvolemic (isovolemic) hyponatremia is most often caused by the syndrome of inappropriate secretion of antidiuretic hormone (SIADH). Common causes of SIADH include some cancers, central nervous system (CNS) and pulmonary disorders, and certain drugs.
![]() Symptoms of hypo- or hypernatremia are usually neurologic and range from weakness, lethargy, restlessness, irritability, and confusion to twitching, seizures, coma, and death. Symptom severity depends on both the magnitude of the change in the serum sodium concentration and the rate at which it changes.
Symptoms of hypo- or hypernatremia are usually neurologic and range from weakness, lethargy, restlessness, irritability, and confusion to twitching, seizures, coma, and death. Symptom severity depends on both the magnitude of the change in the serum sodium concentration and the rate at which it changes.
![]() Treatment goals in patients with either hypo- or hypernatremia should include cautious correction of the serum sodium concentration and, when appropriate, restoration of a normal extracellular fluid (ECF) volume. Too rapid correction of the serum sodium can result in cerebral edema, seizures, neurologic damage, osmotic demyelination syndrome, and possibly death. To minimize the risk of these complications, the serum sodium concentration should be corrected at a rate not to exceed 6 to 12 mEq/L (6 to 12 mol/L) in 24 hours, depending on the rate of change in the serum sodium concentration.
Treatment goals in patients with either hypo- or hypernatremia should include cautious correction of the serum sodium concentration and, when appropriate, restoration of a normal extracellular fluid (ECF) volume. Too rapid correction of the serum sodium can result in cerebral edema, seizures, neurologic damage, osmotic demyelination syndrome, and possibly death. To minimize the risk of these complications, the serum sodium concentration should be corrected at a rate not to exceed 6 to 12 mEq/L (6 to 12 mol/L) in 24 hours, depending on the rate of change in the serum sodium concentration.
![]() Asymptomatic or mildly symptomatic hyponatremia should be managed conservatively with treatment directed at the underlying cause. IV infusion of 0.9% NaCl solution is most often used to correct the serum sodium concentration in patients with moderate to severe symptoms from hypovolemic hypotonic hyponatremia. A 3% NaCl infusion can be cautiously used in patients with moderate to severe symptoms and euvolemic or hypervolemic hypotonic hyponatremia (along with a loop diuretic).
Asymptomatic or mildly symptomatic hyponatremia should be managed conservatively with treatment directed at the underlying cause. IV infusion of 0.9% NaCl solution is most often used to correct the serum sodium concentration in patients with moderate to severe symptoms from hypovolemic hypotonic hyponatremia. A 3% NaCl infusion can be cautiously used in patients with moderate to severe symptoms and euvolemic or hypervolemic hypotonic hyponatremia (along with a loop diuretic).
![]() Hypernatremia is always hypertonic and most commonly occurs when increased water or hypotonic fluid losses are not offset by increased water intake or administration.
Hypernatremia is always hypertonic and most commonly occurs when increased water or hypotonic fluid losses are not offset by increased water intake or administration.
![]() Hypovolemic hypernatremia is relatively common in patients taking loop diuretics. After symptoms of hypovolemia are corrected with 0.9% NaCl solution, free water should be replaced.
Hypovolemic hypernatremia is relatively common in patients taking loop diuretics. After symptoms of hypovolemia are corrected with 0.9% NaCl solution, free water should be replaced.
![]() Patients with central diabetes insipidus (DI) can be treated with desmopressin acetate, with a goal to decrease urine volume to less than 2 L per day while maintaining the serum sodium concentration between 137 and 142 mEq/L (137 and 142 mmol/L). Patients with nephrogenic DI should be treated by correcting the underlying cause, when possible, and sodium restriction in conjunction with a thiazide diuretic to decrease the ECF volume by approximately 1 to 1.5 L.
Patients with central diabetes insipidus (DI) can be treated with desmopressin acetate, with a goal to decrease urine volume to less than 2 L per day while maintaining the serum sodium concentration between 137 and 142 mEq/L (137 and 142 mmol/L). Patients with nephrogenic DI should be treated by correcting the underlying cause, when possible, and sodium restriction in conjunction with a thiazide diuretic to decrease the ECF volume by approximately 1 to 1.5 L.
![]() Edema develops as a primary defect in renal sodium handling or as a response to a decreased effective circulating volume. It is usually first detected in the feet or pretibial areas of ambulatory patients. Pulmonary edema, evidenced by auscultatory crackles, can be life threatening.
Edema develops as a primary defect in renal sodium handling or as a response to a decreased effective circulating volume. It is usually first detected in the feet or pretibial areas of ambulatory patients. Pulmonary edema, evidenced by auscultatory crackles, can be life threatening.
![]() Diuretics are the primary pharmacologic means for minimizing edema and improving organ function. Diuretic resistance often can be overcome by using an increased dose or by using a combination of a loop diuretic and a thiazide or thiazide-like diuretic.
Diuretics are the primary pharmacologic means for minimizing edema and improving organ function. Diuretic resistance often can be overcome by using an increased dose or by using a combination of a loop diuretic and a thiazide or thiazide-like diuretic.
![]() Both blood volume and plasma osmolality are tightly regulated in the human body because they are essential for normal cellular function. Blood volume is a determinant of effective tissue perfusion which is required to deliver oxygen and nutrients to and remove metabolic waste products from tissues. Plasma osmolality, the primary determinant of which is sodium concentration, is an important determinant of intracellular fluid (ICF) volume. Maintenance of normal ICF volume is particularly critical in the brain, which is 80% water, and where alterations, especially rapid changes, can result in significant dysfunction and potentially death.
Both blood volume and plasma osmolality are tightly regulated in the human body because they are essential for normal cellular function. Blood volume is a determinant of effective tissue perfusion which is required to deliver oxygen and nutrients to and remove metabolic waste products from tissues. Plasma osmolality, the primary determinant of which is sodium concentration, is an important determinant of intracellular fluid (ICF) volume. Maintenance of normal ICF volume is particularly critical in the brain, which is 80% water, and where alterations, especially rapid changes, can result in significant dysfunction and potentially death.
Simply put, water balance determines the serum sodium concentration, and sodium balance determines the volume status. Thus, the homeostatic mechanisms for controlling blood volume are focused on controlling sodium balance, and, in contrast, the homeostatic mechanisms for controlling plasma osmolality are focused on controlling water balance. Disorders of sodium and water homeostasis are common, caused by a variety of diseases, conditions, and drugs, and potentially serious. This chapter reviews the etiology, classification, clinical presentation, and therapy for disorders of sodium and water homeostasis.
SODIUM AND WATER HOMEOSTASIS
Hypo- and hypernatremia are syndromes of altered plasma tonicity and cell volume that reflect a change in the ratio of total exchangeable body sodium to total body water (TBW). TBW is distributed primarily into two compartments: the intracellular compartment (ICF; 60% of TBW) and the extracellular compartment or extracellular fluid (ECF; 40% of TBW). Sodium and its accompanying anions (chloride and bicarbonate) comprise more than 90% of the total osmolality of the ECF; whereas ICF osmolality is primarily determined by the concentration of potassium and its accompanying anions (mostly organic and inorganic phosphates). The intra- and extracellular sodium and potassium concentrations are maintained by the sodium–potassium–adenosine triphosphatase (Na+-K+-ATPase) pump. Most cell membranes are freely permeable to water, and thus the osmolalities of the ICF and the ECF are equal.
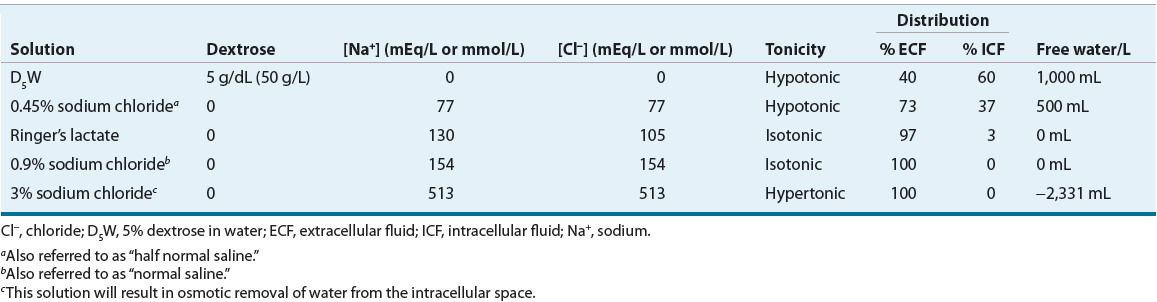
Effective osmoles are solutes that cannot freely cross cell membranes, such as sodium and potassium. The ECF concentration of effective osmoles determines its tonicity, which directly affects the distribution of water between the extra- and intracellular compartments. Addition of an isotonic solution (e.g., 0.9% sodium chloride [NaCl] solution) to the ECF will result in no change in intracellular volume because there will be no change in the effective ECF osmolality. However, addition of a hypertonic solution (e.g., 3% NaCl) to the ECF will result in a decrease in ICF (cell) volume, and addition of a hypotonic solution (e.g., 0.45% NaCl) to the ECF will result in an increase in cell volume. Table 34-1 summarizes the composition of commonly used IV solutions and their respective distribution into the ICF and ECF compartments following IV administration.
TABLE 34-1 Composition of Common IV Solutions
Edelman’s equation defines serum sodium as a function of the total exchangeable sodium and potassium in the body and the TBW: NaS = Natotal body + Ktotal body/TBW, where NaS is the serum sodium concentration; Natotal body is the total body sodium content; Ktotal body is the total body potassium content; and TBW is the total body water in liters.1 The serum sodium concentration is tightly regulated and thus usually varies by no more than 2% to 3%. Regulation of the serum sodium concentration occurs indirectly via mechanisms that control its determinants: plasma osmolality and blood volume. The kidney regulates water excretion through a hypothalamic feedback mechanism, such that the serum osmolality remains relatively constant (275 to 290 mOsm/kg [275 to 290 mmol/kg]) despite day-to-day variations in water intake. Plasma osmolality is primarily determined by the sodium concentration, but serum glucose and blood urea nitrogen (BUN) may contribute significantly at times. Serum osmolality can be estimated as:
![]()
where OsmS is the serum osmolality in mOsm/kg; NaS is the serum sodium concentration in mEq/L; GlucoseS is the serum glucose concentration in mg/dL; and BUN is the blood urea nitrogen concentration in mg/dL. Alternatively, when using SI units the equation becomes:
![]()
where OsmS is the serum osmolality in mmol/kg; NaS is the serum sodium concentration in mmol/L; GlucoseS is the glucose concentration in mmol/L; and BUN is the blood urea nitrogen concentration in mmol/L.
Arginine vasopressin (AVP), commonly known as antidiuretic hormone (ADH), is synthesized in the hypothalamus and released from the posterior pituitary as a result of both osmotic and nonosmotic regulators. When the plasma osmolality increases by 1% to 2% or more AVP is released and binds to the vasopressin 2 (V2) receptors on the basolateral surface of renal tubular epithelial cells, resulting in the insertion of water channels (aquaporin 2) into the apical tubular lumen surface of the cell.2 Water can then pass through the cell into the peritubular capillary space where it is reabsorbed into the systemic circulation. As serum osmolality increases, even as little as 1%, AVP is released and thirst is stimulated. The combined effects of increased water intake and decreased water excretion (kidney’s response to AVP) result in a decrease in the serum osmolality and inhibition of further AVP secretion, once the normal plasma osmolality is restored.
Nonosmotic AVP release occurs when osmoreceptors in the brain detect a 6% to 10% reduction in the effective circulating blood volume or arterial blood pressure. The effective circulating volume is that part of the ECF responsible for organ perfusion. A decrease in the effective circulating volume (more accurately, the pressure associated with that volume) activates arterial baroreceptors in the carotid sinus and glomerular afferent arterioles, resulting in stimulation of the renin–angiotensin system and increased angiotensin II synthesis. Angiotensin II stimulates both nonosmotic AVP release and thirst. This volume stimulus can override osmotic inhibition of AVP release. Conservation of water then restores the effective circulating volume and blood pressure at the expense of producing a decreased serum osmolality and hyponatremia.2 Although hyponatremia and hypernatremia can be associated with conditions of high, low, or normal ECF sodium and volume, both conditions most commonly result from abnormalities of water homeostasis.
HYPONATREMIA
Epidemiology and Etiology
Hyponatremia, usually defined as a serum sodium concentration less than 135 mEq/L (135 mmol/L), is the most common electrolyte abnormality encountered in clinical practice in both adults and children.1,3–6 Although the prevalence is not well established and varies with the patient population studied, it has been estimated to be as high as 28% in patients admitted to an acute care hospital.7 Mild hyponatremia (serum sodium concentration less than 136 mEq/L [136 mmol/L]) was observed in 42.6%, while 6.2% of patients had values less than 126 mEq/L (126 mmol/L), and 1.2% had values less than 116 mEq/L (116 mmol/L). The incidence has been reported to be as high as 21% in patients seen in ambulatory hospital clinics, and 7% in community clinics.7 Drug-induced hyponatremia especially that associated with thiazide diuretics,8,9 and psychotropic medications,10,11 is common. Advancing age (older than 30 years) is also a risk factor for hyponatremia, independent of sex.7
Residents in nursing homes have a twofold higher incidence of hyponatremia than that observed in age-matched, community-dwelling individuals.3 More than 75% of these hyponatremic episodes were precipitated by increased intake of hypotonic oral or IV fluids. Similarly, ingestion of excessive fluid volumes has been identified as a key risk factor in the development of hyponatremia in marathon runners. Although women had a threefold higher rate of hyponatremia, smaller body size and longer racing time, not sex, appear to be the principal factors accounting for the increased incidence.11
Recognition of the high prevalence of hyponatremia is essential because this condition is associated with significant morbidity and mortality.2,12–15 Transient or permanent brain dysfunction can result from either the acute effects of hypoosmolality or too rapid correction of hypoosmolality in patients with hyponatremia. Hyponatremia is predominantly the result of an excess of extracellular water relative to sodium because of impaired water excretion. The kidney normally has the capacity to excrete large volumes of dilute urine after ingestion of a water load. Nonosmotic AVP release, however, can lead to water retention and a drop in the serum sodium concentration, despite a decrease in both serum and intracellular osmolality. The causes of nonosmotic AVP release include hypovolemia, decreased effective circulating volume as seen in patients with chronic heart failure (HF), nephrosis, and cirrhosis. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH), a common cause of hyponatremia, is associated with some oncologic diseases, especially small cell lung cancer, and CNS damage (e.g., head trauma, meningitis). The pathophysiology, clinical features, and management of hyponatremia are detailed below.
Pathophysiology
Hyponatremia can be associated with normal, increased, or decreased plasma osmolality, depending on its cause. Figure 34-1 provides an algorithm for diagnosing patients with hyponatremia. Hyponatremia in patients with normal serum osmolality can be caused by hyperlipidemia or hyperproteinemia. This form of hyponatremia, termed pseudohyponatremia, is an artifact of a specific laboratory method (flame photometry) used to measure serum sodium concentration. This laboratory method is used rarely today, replaced by the use of ion-specific electrodes to measure the serum sodium concentration. If flame photometry is used, the serum volume will be overestimated because the elevated lipids or proteins account for a greater proportion of the total sample volume (Fig. 34-2). Because sodium is distributed in the water component of serum only, the measured serum sodium concentration will be falsely decreased. The measurement of serum osmolality, however, is not significantly affected, leading to a discrepancy between the calculated and measured serum osmolality.
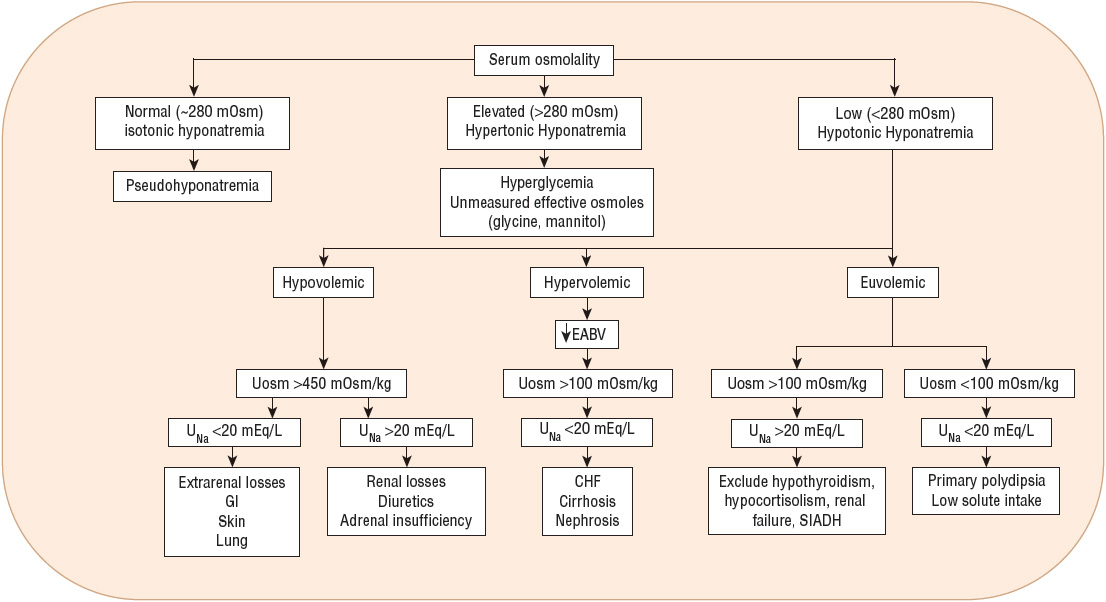
FIGURE 34-1 Diagnostic algorithm for the evaluation of hyponatremia. (CHF, congestive heart failure; SIADH, syndrome of inappropriate secretion of antidiuretic hormone; UNa, urine sodium concentration [values in mEq/L are numerically equivalent to mmol/L]; Uosm, urine osmolality [values in mOsm/kg are numerically equivalent to mmol/kg].)
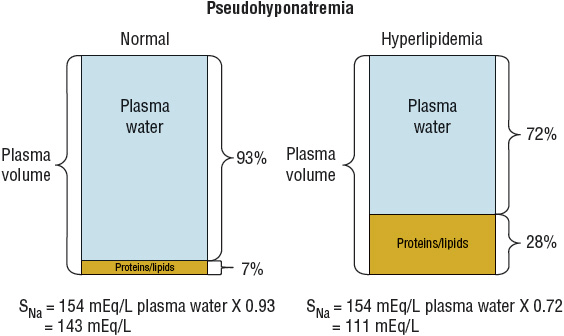
FIGURE 34-2 Elevated lipids or proteins result in a larger discrepancy between the volume of the sample and plasma water, leading to a falsely low measurement of the serum sodium concentration when using the method of flame photometry. (SNa, serum sodium concentration [values in mEq/L are numerically equivalent to mmol/L].)
Hyponatremia associated with an increased serum osmolality, hypertonic hyponatremia, suggests the presence of excess, effective osmoles (other than sodium) in the ECF. This type of hyponatremia is most frequently encountered in patients with hyperglycemia. The elevated glucose concentration provides effective plasma osmoles, resulting in diffusion of water from the cells (ICF) into the ECF, thereby decreasing the ICF, expanding the ECF, and decreasing the serum sodium concentration. In fact, this relationship can be quantified: for every 100 mg/dL (5.6 mmol/L) increase in the serum glucose concentration, the serum sodium concentration decreases by 1.7 mEq/L (1.7 mmol/L) or 0.29 mmol/L for every 1 mmol/L decrease, and the serum osmolality increases by 2 mOsm/kg (2 mmol/kg). This correction is only a rough estimate because the decrease in the serum sodium concentration may vary significantly with any degree of hyperglycemia.15 Other substances such as mannitol, glycine, and sorbitol that do not cross cell membranes provide effective osmoles and can also cause hypertonic hyponatremia. The presence of any one of these unmeasured osmoles should be suspected in patients with hypertonic hyponatremia if there is a significant osmolal gap, defined as the difference between the measured and calculated plasma osmolality.
Hyponatremia associated with decreased plasma osmolality, hypotonic hyponatremia, is the most common form of hyponatremia and has many potential causes (see Table 34-2). Clinical assessment of ECF volume is an important step in the diagnostic evaluation of a patient with hypotonic hyponatremia. Categorization of these patients into one of three groups (decreased, increased, or clinically normal ECF volume) is essential in identifying the pathophysiologic mechanisms responsible for the hyponatremia and developing an appropriate treatment plan.
TABLE 34-2 Characteristics of Hypotonic Hyponatremic States
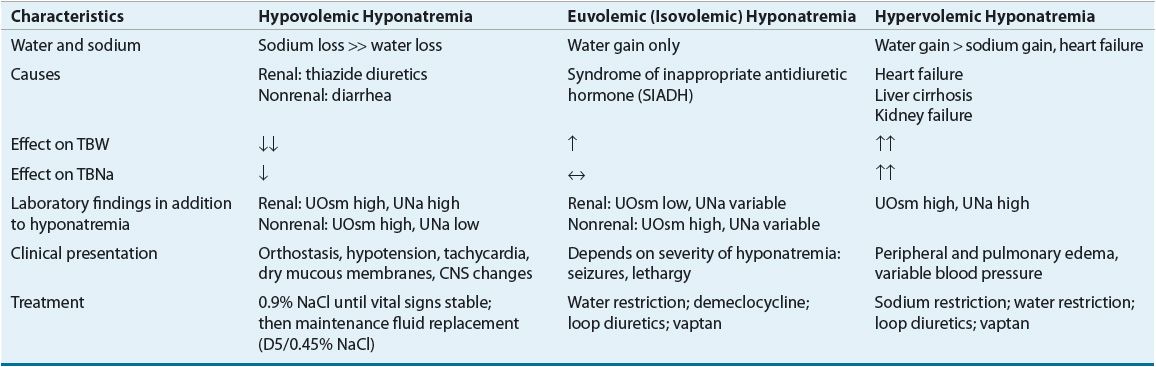
Hypovolemic Hypotonic Hyponatremia
Most patients with ECF volume contraction lose fluids that are hypotonic relative to plasma and thus can become transiently hypernatremic. This includes patients with fluid losses caused by diarrhea, excessive sweating, and diuretics. This transient hypernatremic hyperosmolality results in osmotic AVP release and stimulation of thirst. If sodium and water losses continue, the resultant hypovolemia results in more AVP release. Patients who then drink water (a hypotonic fluid) or who are given hypotonic IV fluids retain water, and hyponatremia develops. These patients typically have a urine osmolality greater than 450 mOsm/kg (450 mmol/kg), reflecting AVP action and formation of a concentrated urine. The urine sodium concentration is less than 20 mEq/L (20 mmol/L) when sodium losses are extrarenal, as in patients with diarrhea, and greater than 20 mEq/L (20 mmol/L) in patients with renal sodium losses, as occurs with thiazide diuretic use or in adrenal insufficiency.17
![]() Hypotonic hyponatremia is relatively common in patients taking thiazide diuretics.9,18 Thiazide-induced hyponatremia is usually mild and relatively asymptomatic; only occasionally is it severe and symptomatic.18 Hyponatremia typically develops within 2 weeks of therapy initiation, but can occur later in therapy, particularly after dosage increases or if other causes of hyponatremia develop.18 The elderly, especially women, are at the greatest risk for thiazide diuretic-induced hyponatremia.
Hypotonic hyponatremia is relatively common in patients taking thiazide diuretics.9,18 Thiazide-induced hyponatremia is usually mild and relatively asymptomatic; only occasionally is it severe and symptomatic.18 Hyponatremia typically develops within 2 weeks of therapy initiation, but can occur later in therapy, particularly after dosage increases or if other causes of hyponatremia develop.18 The elderly, especially women, are at the greatest risk for thiazide diuretic-induced hyponatremia.
The mechanism of thiazide-induced hyponatremia is likely related to the balance of its direct and indirect effects. Thiazide diuretics exert their effects by blocking sodium reabsorption in the distal tubules of the renal cortex, thereby increasing sodium and water removal from the body. The resulting decrease in effective circulating volume stimulates AVP release, resulting in increased free water reabsorption in the collecting duct, as well as increased water intake because of stimulation of thirst. Hyponatremia develops when the net result of these effects is the loss of more sodium than water.
Conversely, hyponatremia occurs infrequently with loop diuretics due to their different sites of action. Loop diuretics exert their diuretic effect by blocking sodium reabsorption in the ascending limb of the loop of Henle. This action decreases medullary osmolality. Thus, when the loop diuretics decrease effective circulating volume and stimulate AVP release, less water reabsorption occurs in the collecting ducts than would occur if the osmolality of the renal medulla were normal. Thiazide diuretics do not alter medullary osmolality because their site of action is in the renal cortex not the medulla. In addition, most loop diuretics have a shorter half-life than the thiazides, and patients can usually replete the urinary sodium and water losses prior to taking the next dose, thereby minimizing AVP stimulation.
Euvolemic Hypotonic Hyponatremia
![]() Euvolemic (isovolemic) hypotonic hyponatremia is associated with a normal or slightly decreased ECF sodium content and increased TBW and ECF volume. The increase in ECF volume is usually not sufficient to cause peripheral or pulmonary edema or other signs of volume overload, and thus patients appear clinically euvolemic. Euvolemic hyponatremia is most often caused by SIADH.
Euvolemic (isovolemic) hypotonic hyponatremia is associated with a normal or slightly decreased ECF sodium content and increased TBW and ECF volume. The increase in ECF volume is usually not sufficient to cause peripheral or pulmonary edema or other signs of volume overload, and thus patients appear clinically euvolemic. Euvolemic hyponatremia is most often caused by SIADH.
In SIADH, water intake exceeds the kidney’s capacity to excrete water, either because of increased AVP release via nonosmotic and/or nonphysiologic processes or enhanced sensitivity of the kidney to AVP. In patients with SIADH, the urine osmolality is generally greater than 100 mOsm/kg (100 mmol/kg), and the urine sodium concentration is usually greater than 20 mEq/L (20 mmol/L) due to the ECF volume expansion (Table 34-2).
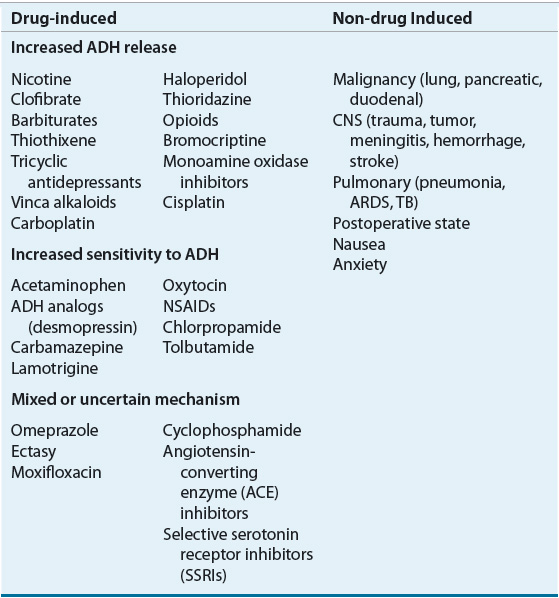
The most common causes of SIADH include tumors such as small cell lung or pancreatic cancer, CNS disorders (e.g., head trauma, stroke, meningitis, pituitary surgery), and pulmonary disease (e.g., tuberculosis, pneumonia, acute respiratory distress syndrome). Patients with kidney and adrenal insufficiency or hypothyroidism can also present with euvolemic hyponatremia, and the evaluation of patients with suspected SIADH should always include consideration of these disorders as the etiology. A variety of drugs can cause SIADH by enhancing AVP release, the effect of AVP on the kidney, or by other unknown mechanisms10,14,15,20 (Table 34-3). The differential diagnosis of euvolemic hypotonic hyponatremia also includes primary or psychogenic polydipsia. Patients with this disorder drink more water (usually more than 20 L/day) than the kidneys can excrete as solute-free water. However, unlike in SIADH, AVP secretion is suppressed, resulting in a urine osmolality that is less than 100 mOsm/kg (100 mmol/kg). The urine sodium is typically low (less than 15 mEq/L [15 mmol/L]) as a result of dilution.11 Hyponatremia can develop even with more modest water intakes in patients who are ingesting very low-solute diets.
TABLE 34-3 Potential Causes of SIADH
Hypervolemic Hypotonic Hyponatremia
Hyponatremia associated with ECF volume expansion occurs in conditions in which the kidney’s sodium and water excretion are impaired. Patients with cirrhosis, HF, or nephrotic syndrome have an expanded ECF volume and edema, but a decreased effective arterial blood volume (EABV). This decreased volume results in renal sodium retention, and eventually ECF volume expansion and edema. At the same time, there is nonosmotic stimulation of AVP release and water retention in excess of sodium retention, which perpetuates the hyponatremic state.
Clinical Controversy…
Clinical Presentation
![]() The clinical presentation of patients with hyponatremia is summarized in Table 34-4. Patients with chronic (defined as lasting longer than 48 hours), mild hyponatremia (serum sodium concentration 125 to 134 mEq/L [125 to 134 mmol/L]) are usually asymptomatic, with hyponatremia being discovered incidentally when serum electrolytes are measured for other purposes.21 However, mild symptoms of hyponatremia are frequently unnoticed by both clinicians and patients.22 Chronic, mild hyponatremia is associated with impairment of attention, posture, and gait, all of which contribute to a substantially increased fall risk. Even “asymptomatic” patients, when formally tested, have impaired attention and gait to a degree that is comparable to symptoms seen with a blood alcohol level of 0.06% (13 mmol/L).23,24
The clinical presentation of patients with hyponatremia is summarized in Table 34-4. Patients with chronic (defined as lasting longer than 48 hours), mild hyponatremia (serum sodium concentration 125 to 134 mEq/L [125 to 134 mmol/L]) are usually asymptomatic, with hyponatremia being discovered incidentally when serum electrolytes are measured for other purposes.21 However, mild symptoms of hyponatremia are frequently unnoticed by both clinicians and patients.22 Chronic, mild hyponatremia is associated with impairment of attention, posture, and gait, all of which contribute to a substantially increased fall risk. Even “asymptomatic” patients, when formally tested, have impaired attention and gait to a degree that is comparable to symptoms seen with a blood alcohol level of 0.06% (13 mmol/L).23,24
TABLE 34-4 Clinical Presentation of Hyponatremia
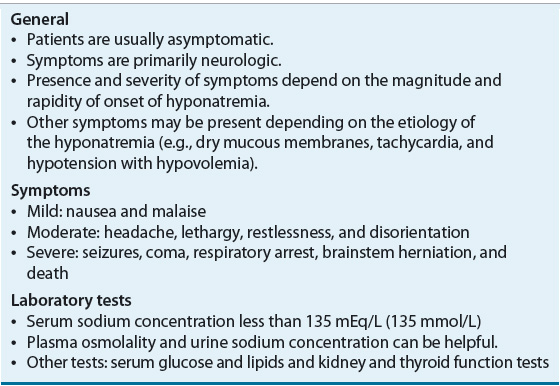
Patients with moderate (serum sodium concentration 115 to 124 mEq/L [115 to 124 mmol/L]), severe (serum sodium concentration 110 to 114 mEq/L [110 to 114 mmol/L]), or rapidly developing hypotonic hyponatremia often present with a range of neurologic symptoms resulting from hypoosmolality-induced brain cell swelling. Classic neurologic symptoms include nausea, malaise, headache, lethargy, restlessness, and disorientation. In severe cases, seizures, coma, respiratory arrest, brainstem herniation, and death can occur.
The presence of these symptoms and their severity depend on both the magnitude of the hyponatremia and the rate at which the hyponatremia develops. The magnitude of the hyponatremia is important because serum osmolality decreases in direct proportion to the serum sodium concentration, and water movement into brain cells increases as serum osmolality decreases. The rate of change of the serum osmolality is an important factor because brain cells are able to adjust their intracellular osmolality to minimize cellular volume changes in response to volume changes, but time is required for this adaptation to occur.25 When a decline in plasma osmolality causes water movement into brain cells, inorganic Cl– and K+, and organic osmolytes, such as taurine, glutamate, and myoinositol, move out of the cells to decrease intracellular osmolality and minimize intracellular water shifts.26 Organic osmolytes, such as myoinositol, a osmotically active substances contribute substantially to controlling intracellular osmolality in the brain without directly altering cellular function.25,26 The various components of this adaptive mechanism occur over different time frames, with sodium and potassium efflux occurring within minutes to several hours and organic osmolyte efflux occurring within hours to several days.25,26 Maximal compensation for decreased plasma osmolality typically requires up to 48 hours. Thus, acute changes in plasma osmolality are more likely to be associated with symptoms. Concurrent respiratory failure and hypoxemia increase the risk of adverse neurologic outcomes because hypoxemia diminishes the brain’s capacity to actively transport solute out of cells, leading to a higher incidence of cerebral edema.25,26 Children and women have poorer clinical outcomes than adults and males, respectively. For example, post-menopausal women have a 25-fold higher risk of death or permanent neurological damage with acute hypervolemic hypotonic hyponatremia than men.27 Hyponatremia is a severe risk factor for morbidity and mortality in patients with HF and cirrhosis.2
In addition to neurologic symptoms, patients with hypovolemic hyponatremia can present with signs and symptoms of hypovolemia, including dry mucous membranes, decreased skin turgor, tachycardia, decreased jugular venous pressure, hypotension, and orthostatic hypotension. These findings often are helpful in identifying the type of hyponatremia present.
![]() The brain’s adaptation to a chronic change in the plasma osmolality leads to development of neurologic symptoms if hyponatremia (hypoosmolality) is corrected too rapidly. The combination of the adaptive decrease in intracellular osmolality and rapid increase in serum osmolality results in excessive movement of water out of the brain cells and ICF volume depletion. Too rapid correction of the serum sodium concentration can lead to an acute decrease in brain cell volume, which contributes to the pathogenesis of osmotic demyelination syndrome (ODS),2,28 also known as central pontine myelinolysis, because the demyelinated lesions, which appear on magnetic resonance imaging, most often occur in the central pons; however, it can extend to extrapontine structures.1 Patients with this complication might develop hyperreflexia, para- or quadriparesis, parkinsonism, pseudobulbar palsy, locked-in syndrome (a condition in which a patient is aware and awake but cannot move or communicate verbally due to complete paralysis of nearly all voluntary muscles in the body except for the eyes), or death approximately 1 to 7 days after treatment.1,12,29 Patients with a significant degree of cerebral adaptation (e.g., chronic serum sodium concentration less than 110 mEq/L [110 mmol/L]) to hypotonic hyponatremia are at highest risk of developing this syndrome because these patients have lower intracellular osmolalities at the initiation of therapy, resulting in a greater decrease in intracellular volume in brain cells when the plasma osmolality is raised too rapidly.28 Other conditions that increase the risk of ODS include alcoholism, liver failure, orthotopic liver transplantation, potassium depletion, and malnutrition. Thus, if duration of hyponatremia is unknown; then it is generally safer to treat as if it is chronic when developing an initial treatment plan.1
The brain’s adaptation to a chronic change in the plasma osmolality leads to development of neurologic symptoms if hyponatremia (hypoosmolality) is corrected too rapidly. The combination of the adaptive decrease in intracellular osmolality and rapid increase in serum osmolality results in excessive movement of water out of the brain cells and ICF volume depletion. Too rapid correction of the serum sodium concentration can lead to an acute decrease in brain cell volume, which contributes to the pathogenesis of osmotic demyelination syndrome (ODS),2,28 also known as central pontine myelinolysis, because the demyelinated lesions, which appear on magnetic resonance imaging, most often occur in the central pons; however, it can extend to extrapontine structures.1 Patients with this complication might develop hyperreflexia, para- or quadriparesis, parkinsonism, pseudobulbar palsy, locked-in syndrome (a condition in which a patient is aware and awake but cannot move or communicate verbally due to complete paralysis of nearly all voluntary muscles in the body except for the eyes), or death approximately 1 to 7 days after treatment.1,12,29 Patients with a significant degree of cerebral adaptation (e.g., chronic serum sodium concentration less than 110 mEq/L [110 mmol/L]) to hypotonic hyponatremia are at highest risk of developing this syndrome because these patients have lower intracellular osmolalities at the initiation of therapy, resulting in a greater decrease in intracellular volume in brain cells when the plasma osmolality is raised too rapidly.28 Other conditions that increase the risk of ODS include alcoholism, liver failure, orthotopic liver transplantation, potassium depletion, and malnutrition. Thus, if duration of hyponatremia is unknown; then it is generally safer to treat as if it is chronic when developing an initial treatment plan.1
TREATMENT
![]() The following principles serve as general guidelines for the treatment of patients with hyponatremia:1,18,21,30 (a) It is important for both short- and long-term management to treat the underlying cause of hyponatremia. (b) Appropriate treatment of hypotonic hyponatremia requires balancing the risks of hyponatremia versus the risk of ODS. In general, patients who acutely developed moderate to severe hyponatremia and/or patients who have severe symptoms are at greatest risk and potentially benefit most from more rapid correction of hyponatremia. (c) Correction of hypovolemic hypotonic hyponatremia is usually best accomplished with 0.9% NaCl solution, as these patients have both sodium and water deficits. (d) Active correction of euvolemic and hypervolemic hypotonic hyponatremia in patients who do not require rapid correction is usually best accomplished by water restriction. Demeclocycline, AVP vasopression 2 receptor antagonists (vaptans), or 0.9% NaCl solution plus a loop diuretic (furosemide, bumetanide) can be used if the initial response to water restriction is not adequate. (e) In patients with severe symptoms, 3% NaCl solution (possibly combined with a loop diuretic) should initially be used to more rapidly correct the hyponatremia. A loop diuretic such as furosemide can be administered concurrently with 3% NaCl to enhance the serum sodium correction by increasing free water excretion. (f) Long-term management will be required for patients in whom the underlying cause of hyponatremia cannot be corrected. Depending on the cause, water restriction, increasing sodium intake, and/or the use of an AVP antagonist (vaptan) can be used. Application of these principles to the treatment of patients with various forms of hypotonic hyponatremia is discussed in the following sections.
The following principles serve as general guidelines for the treatment of patients with hyponatremia:1,18,21,30 (a) It is important for both short- and long-term management to treat the underlying cause of hyponatremia. (b) Appropriate treatment of hypotonic hyponatremia requires balancing the risks of hyponatremia versus the risk of ODS. In general, patients who acutely developed moderate to severe hyponatremia and/or patients who have severe symptoms are at greatest risk and potentially benefit most from more rapid correction of hyponatremia. (c) Correction of hypovolemic hypotonic hyponatremia is usually best accomplished with 0.9% NaCl solution, as these patients have both sodium and water deficits. (d) Active correction of euvolemic and hypervolemic hypotonic hyponatremia in patients who do not require rapid correction is usually best accomplished by water restriction. Demeclocycline, AVP vasopression 2 receptor antagonists (vaptans), or 0.9% NaCl solution plus a loop diuretic (furosemide, bumetanide) can be used if the initial response to water restriction is not adequate. (e) In patients with severe symptoms, 3% NaCl solution (possibly combined with a loop diuretic) should initially be used to more rapidly correct the hyponatremia. A loop diuretic such as furosemide can be administered concurrently with 3% NaCl to enhance the serum sodium correction by increasing free water excretion. (f) Long-term management will be required for patients in whom the underlying cause of hyponatremia cannot be corrected. Depending on the cause, water restriction, increasing sodium intake, and/or the use of an AVP antagonist (vaptan) can be used. Application of these principles to the treatment of patients with various forms of hypotonic hyponatremia is discussed in the following sections.
Desired Outcome
Regardless of the type or cause of hyponatremia, the goals of treatment for all patients are to resolve the underlying cause of the sodium and ECF volume imbalance, if possible, and to safely correct the sodium and water derangements. The treatment plan for patients with hyponatremia depends on the underlying cause of the hyponatremia and the severity of the patient’s symptoms. Patients with an acute onset of hyponatremia or severe symptoms require more aggressive therapy to correct the hypotonicity. The initial goal for these patients is to increase plasma tonicity just enough to control severe symptoms; this typically requires only a small increase (5%) in serum sodium concentration. Once severe symptoms have abated, then continued correction of the serum sodium concentration should be achieved at a controlled rate. Patients who are asymptomatic or who have only mild to moderate symptoms do not require rapid correction of the serum sodium concentration. Treatment is dictated by the underlying etiology. In all cases the goal is to avoid an increase in the serum sodium concentration of more than 12 mEq/L (12 mmol/L) in 24 hours or 0.5 mEq/L (0.5 mmol/L) per hour.1,2,21,30 However, because of the usual uncertainty regarding duration of hyponatremia, correction of no more than 6 to 8 mEq/L (6 to 8 mmol/L) or 0.33 mEq/L/h (0.33 mmol/L/h) is prudent to avoid ODS.1
ACUTE OR SEVERELY SYMPTOMATIC HYPOTONIC HYPONATREMIA
A patient who has or is at high risk of experiencing severe symptoms caused by hyponatremia should receive either 3% NaCl (513 mEq/L [513 mmol/L]) or 0.9% NaCl (154 mEq/L [154 mmol/L]) solution until severe symptoms resolve.1,3,18,22,32 Resolution of severe symptoms frequently requires only a small (~5%) increase in serum sodium concentration; although, some clinicians suggest that the initial safe target should be a serum sodium concentration of approximately 120 mEq/L (120 mmol/L).3,33 The relative concentrations of urine sodium and potassium (osmotically effective urine cations) must be compared with those of the infusate in planning a treatment regimen for patients with hypotonic hyponatremia. For the serum sodium concentration to increase after infusion of a sodium chloride solution, the sodium concentration of the infusate must exceed the sum of the urinary sodium and potassium concentrations to produce an effective net free-water excretion.
Patients with SIADH often have urinary concentrations of osmotically effective cations that exceed the sodium concentration of 0.9% NaCl. In this case, use of isotonic sodium chloride can actually worsen hyponatremia.31 These patients should be preferentially treated with 3% NaCl solution. The relatively high urinary sodium concentration in patients with SIADH is due to ECF expansion, which minimizes sodium reabsorption along the nephron. When the urine osmolality exceeds 300 mOsm/kg (300 mmol/kg), it is generally advisable to administer an IV loop diuretic, not only to increase solute-free water excretion but also to prevent volume overload, which can result from infusion of hypertonic sodium chloride. IV furosemide, 20–40 mg every 6 hours, or bumetanide, 0.5 to 1 mg/dose every 2 to 3 hours for two doses, is generally sufficient to prevent volume overload and to decrease the urinary concentration of osmotically active cations to less than 150 mEq/L (150 mmol/L). If intermittent loop diuretic doses are not sufficient to manage edema, then continuous infusions have been used. Furosemide, 20 to 40 mg, given IV, followed by a 10 to 40 mg/h infusion, or bumetanide 1 mg given IV followed by a 0.5 to 2 mg/h infusion have been used.
Patients with hypovolemic hypotonic hyponatremia can be treated with 0.9% NaCl solution. In contrast to patients with SIADH, patients with this condition avidly reabsorb sodium throughout the nephron because the effective circulating blood volume is decreased. Thus, the urine sodium concentration is often less than 20 mEq/L (20 mmol/L), substantially less than the sodium content of 0.9% NaCl solution. While the use of 3% NaCl solution will correct hyponatremia in these patients, it will not correct the hypovolemia; thus, its use should be reserved for patients with severe symptoms requiring very rapid correction of the serum sodium concentration.
Acute hypervolemic hypotonic hyponatremia is particularly problematic to manage because the sodium and volume needed to minimize the risk of cerebral edema or seizures can worsen already compromised liver, heart, or kidney function. These patients generally should be treated with 3% NaCl and initiation of fluid (water) restriction. Loop diuretic therapy will also likely be required to facilitate urinary free water excretion.
Determination of a Sodium Chloride Infusion Regimen
Several methods for determining the correct sodium chloride solution infusion regimen for a patient with hyponatremia have been proposed.1,2,18,29,32,33 These empiric approaches provide only an initial estimate of the correct infusion regimen. More complex equations have been derived, but improved outcomes using these equations have not been demonstrated.18,29
One common approach to acute treatment of hyponatremia is to estimate the change in serum sodium concentration resulting from the infusion of 1 L of 3% or 0.9% NaCl solution. An example of this approach is shown in Box 34-1. Another method involves calculating the sodium deficit, then replacing one-third of the deficit in the first 6 hours with the remaining two thirds being replaced over the following 24 to 48 hours. Sodium deficit can be calculated using the following equation:
![]()
where NaD is the goal serum sodium (usually 125 to 130 mEq/L [125 to 130 mmol/L] to avoid too rapid correction); NaS is the patient’s current serum sodium concentration; and, TBW is the patient’s current total body water calculated as shown in Box 34-1. The appropriate infusion volume for a given patient can then be estimated using the desired proportion of the estimated change that would result from a 1-L infusion or the amount of fluid needed to provide the calculated sodium deficit. The final step is to calculate an appropriate infusion rate for the calculated volume that will control the rate of increase of the serum sodium concentration to 6 to 12 mEq/L (6 to 12 mmol/L) in 24 hours (Box 34-1). Using desmopressin in combination with 3% NaCl solution to minimize the risk of treating hyponatremia has been suggested but is generally not recommended.1



