Chapter 12 Diseases of the anogenital skin
Introduction
Over the years, various unsatisfactory classifications have been devised for vulval disorders.1 Terms such as squamous cell hyperplasia are clinically meaningless and at best histologically descriptive. There is probably no need for a separate classification for cutaneous disorders that affect the vulval skin and mucosa. The terminology for vulval intraepithelial neoplasia (VIN) and penile intraepithelial neoplasia (PIN) are discussed later. Extramammary Paget’s disease and in situ melanoma are no longer included in the spectrum of VIN.
Normal female anatomy
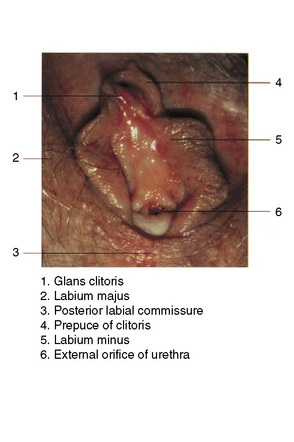
The vulva comprises the mons pubis, the labia majora and minora, the vestibule, the clitoris (including the prepuce and frenulum), the glands of Bartholin and Skene, the hymen, posterior commissure, fossa navicularis, introitus and the external urethral orifice (Fig. 12.1).
Labia majora and perianal skin
Histological features
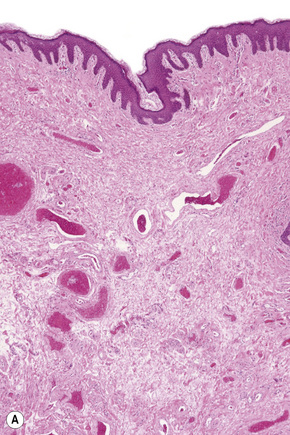
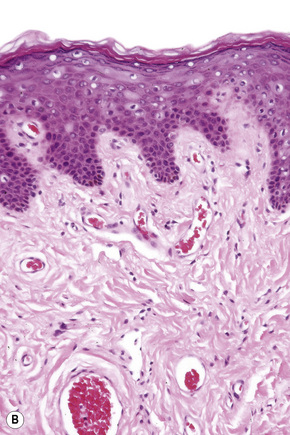
These sites most closely resemble skin from other regions of the body since the epithelium is keratinized and stratified and adnexal structures are represented. The epithelium of the labia majora normally contains occasional lymphocytes, which are found in very small numbers around the superficial dermal vasculature. In the labia majora, sebaceous glands are present in association with hair follicles and both eccrine and apocrine glands are typically present. On the medial aspect of the labia majora the hair follicles become modified and there is often no hair seen although sebaceous glands are still present (Fig. 12.2). Some may open directly onto the epithelium (Fordyce spots, see below). In the deep dermis, a layer of smooth muscle known as the tunica dartos labialis is seen. The subcutaneous tissue of the labia majora tends to be prominent in women of reproductive age but decreases in amount after the menopause. The labia majus contains a long smooth muscle called the cremaster. The skin of the perianal area shows numerous terminal hairs with sebaceous glands.
Vestibule
Histological features
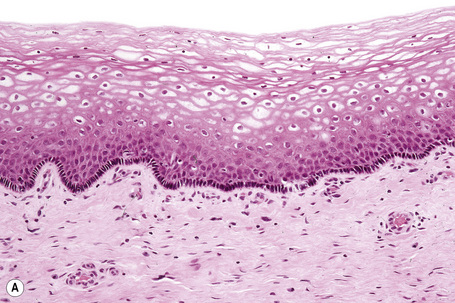
The epithelium is stratified and nonkeratinized, i.e., it is a mucosa (Fig. 12.3). Both the vagina and the urethra open into the vestibule. Bartholin’s glands are located deep to the posterior part of the labia majora. They represent the equivalent of Cowper’s glands or the bulbourethral glands in the male. The ducts of Bartholin and Skene’s glands and the minor vestibular glands open into the vestibule
Normal male anatomy
The prepuce has been present in primates for at least 65 million years and indeed this may be an underestimate, 100 million years having been suggested. The female counterpart is the clitoral hood. Only 4% of boys have a retractable foreskin at birth, 15% at six months, 50% at 1 year and 80–90% at 3 years.1 The separation of the mucosa of the prepuce and glans is usually complete by 17 years.2–4 The prepuce is composed of specialized protective and erogenous tissue. The protection it affords is both physical and immunological.4 Its structures (e.g., the penile dartos muscle and the corpuscular receptor-rich ridged band) and secretions are held to be important for normal function. The foreskin is of variable length and retractability in uncircumcised men.
Histological features
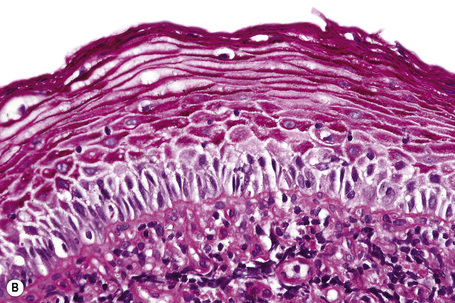
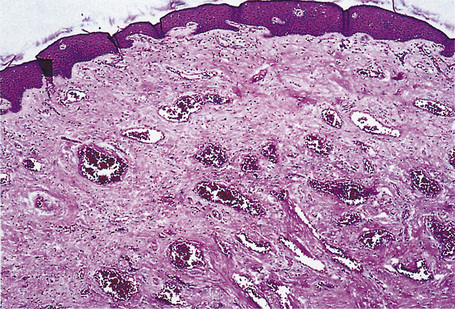
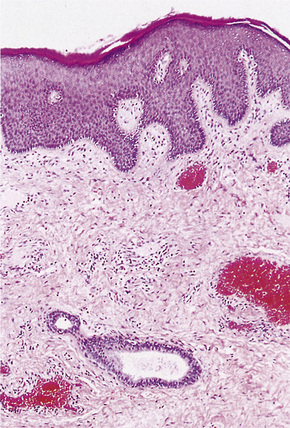
The pattern of keratinization of the epithelium varies throughout the anogenital area most markedly in the transition from true urothelium to true skin. There is controversy over the definition of ‘mucosa’ 5,6 but there is no doubt that the penile urethra does possess a true mucosa, while the glans of the circumcised male does not; the glans and inner prepuce of the uncircumcised male probably does not display mucosa but the outer prepuce does. There are several epithelial transition zones.7 The proximal (pre-prostatic and prostatic) urethra is lined by transitional epithelium, the membranous spongy penile urethra by pseudostratified columnar epithelium. The distal penile urethra is lined by stratified or pseudo-stratified squamous epithelium incorporating a single layer of columnar cells at the surface and contains Littre’s glands, the distal 5–6 mm of penile urethra, which includes the navicular fossa manifests a 6–10 cell layer of nonkeratinizing squamous epithelium, contains no adnexae and is continuous with the epithelium of the uncircumcised glans (which may show some thin keratinization, although some accounts state that the glans is not keratinized in the uncircumcised) and inner preputial epithelium; the outer preputial epithelium is identical to skin.7 Just as there is wide variability in the size and shape of the navicular fossa, the site of the epithelial transition zones and probably the degree of keratinization of the glans and the disposition of adnexa may vary. Presumed multipotential, mucous secreting cells have been described in the perimeatal epithelium8 and mucinous metaplasia of glans and foreskin have also been observed.9,10 Also, the final transition will depend on the length of the foreskin: it is our experience that male genital lichen sclerosus has a greater predilection for the male with a longer foreskin (as does penile cancer11). The wide spectrum of differentiation of the male urogenital tract is accompanied by changes in the expression of epithelial cytokeratins, but this is relatively unexplored territory.12 Normal regional histology is illustrated in Figures 12.4–12.9.
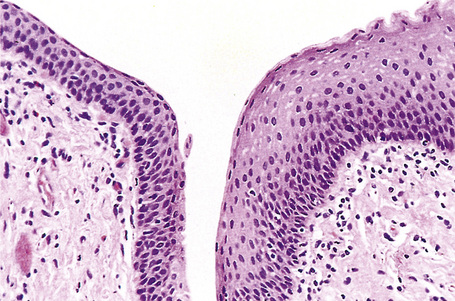
Fig. 12.8 Anal transitional zone (ATZ). Transition from ATZ epithelium (left) to squamous zone (right). H&E.
Reproduced with permission from Sternberg, SS, ed. Histology for Pathologists, 2nd edition. Philadelphia: Lippincott, Williams and Wilkins, 1997.
Embryology
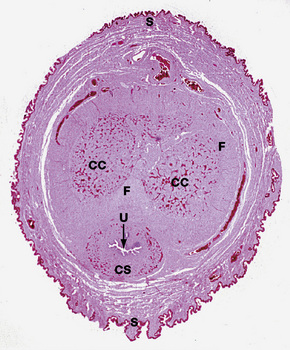
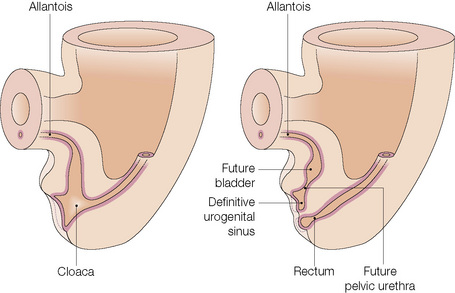
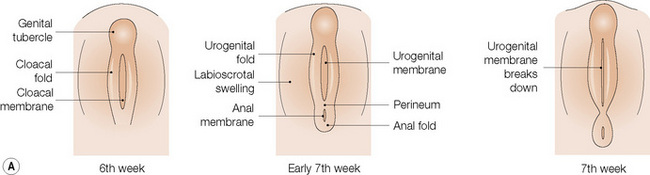
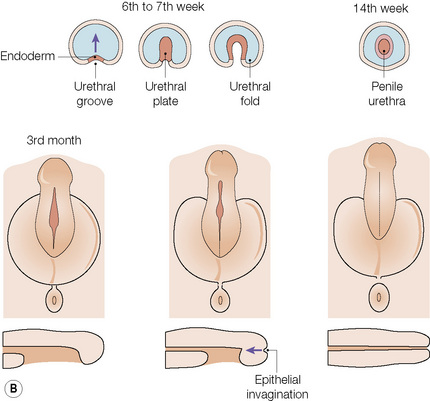
The anatomical position, the arrangement of all of the structures in the area, the normal histology and some of the histopathology are explained by the embryology (Figs 12.10, 12.11).1,2 Around the third week of fetal development, mesenchymal cells derived from the primitive streak form ridges of tissue around the cloacal membrane. These cloacal folds are joined anteriorly and cranially and form the genital tubercle. Posteriorly and caudally, they are partially joined to form an annulus. The underlying cloacal membrane is now subdivided into urogenital and anal membranes craniocaudally by about 6 weeks. During the same period, lateral genital swellings develop that will form either the scrotum or labia majora.
From this point differentiation of the external genitalia occurs in a gender-specific manner, driven in the male by fetal testicular androgens. The genital tubercle lengthens, creating a urethral groove. This eventually becomes the urethral canal when the folds fuse at about 12 weeks. The penile urethra has an epithelium derived therefore from endoderm. It is incomplete cranially where the glans has developed from the genital tubercle. The glandular urethra, the navicular fossa and the meatus are derived from an invading cord of ectoderm that eventually becomes canalized. There is wide variability in the size and shape of the navicular fossa in men, testifying to the variable consequences of this embryological process. The scrotal swellings fuse posteriorly at about 14 weeks. They are not occupied by the testes until about the time of birth. The cloacal membrane is where ectodermal and endodermal tissues are in direct apposition caudally. The separation into urogenital membrane and anal membrane with the formation of the perineum at about 7 weeks is due to the separation of the cloacal portion of the hindgut by the urorectal septum growing caudally between the allantois anteriorly and the hindgut and partitioning the cloaca into the urogenital sinus anteriorly and the anorectal canal posteriorly. The anal membrane disintegrates at about 9 weeks to open into an ectodermal anal pit formed in the posterior cloacal (anal) folds. The prepuce 3,4 is formed by a midline collision of ectoderm, neuroectoderm and mesenchyme, resulting in a pentalaminar structure consisting of (inner) squamous mucosal epithelium, lamina propria, dartos muscle, dermis and glabrous skin (outer). The preputial fold progressively extends but there is also an ingrowth of a cellular lamella. It then fuses with the mucosa of the glans. At birth, the prepuce is still developing histologically and it is usually incompletely separated from the glans.
Pubic hair
Pubic hair appears during puberty as vellus hair that is focally replaced by terminal hair. Men have a different pattern of pubic hair than women but in practice it is one of degree. The distribution of hair and pubic hair varies widely between men.1 Generally, the abdominal wall, pubic mound, groins, scrotum and perineum are hairy but the natal cleft, perianal skin, distal penile shaft, prepuce and glans are hairless.
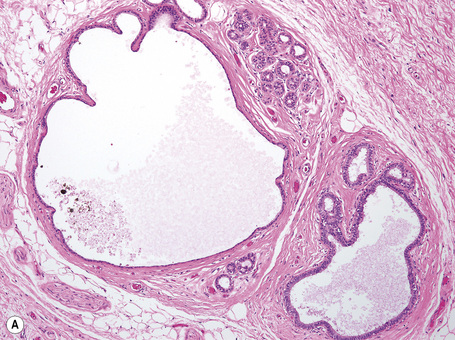
Anogenital ‘sweat’ glands
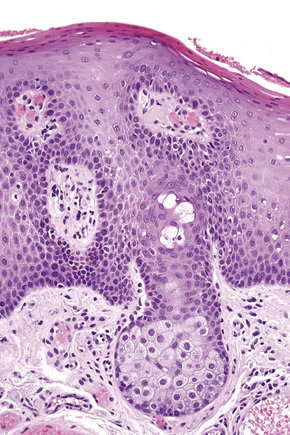
Anogenital ‘sweat’ glands, which were first described by Van der Putte, are found in anogenital skin, mainly in the interlabial sulci.1,2 They share morphological and histological features of eccrine, apocrine and mammary glands, making categorization difficult (Fig. 12.12). Superficially, an excretory duct opens directly onto the skin. A deeper coiled or a long straight duct gives rise to several sac-like invaginations forming small glands, which typically extend deeper than the apocrine or eccrine glands. The anogenital sweat glands are lined by simple columnar epithelium surrounded by a layer of myoepithelial cells (Fig. 12.12). It has been suggested that adnexal tumors arising in the genital skin derive from or show differentiation towards the anogenital glands and, in a number of cases, lesions are very similar to tumors occurring in the breast.3,4
Normal Variants
Circumcision
The commonest variation is the presence or absence of the foreskin. Circumcision is the oldest elective operation, with evidence of its practice in Ancient Egypt between 2400 and 3000 bc.1 The operation has been performed for religious, cultural or medical reasons throughout history.2 It has been estimated that globally 25% of men have been circumcised.3 The prevalence of circumcision in any population reflects racial, religious, cultural and medical differences. The risks and benefits of neonatal circumcision have been the focus of much debate.4–6 Importantly, circumcision protects men from cancer of the penis, urinary tract and sexually transmitted infections including HIV and genital dermatoses.7 However, the incidence of penile cancer is low in Japan and Denmark where circumcision is rare,8 and therefore other factors are important in its pathogenesis .
Circumcision is indispensible in the management of diseases of the penis and foreskin, including dermatological conditions, and is being investigated for the control of HIV infection. Although the long-held consensus is that there is little evidence of significant adverse effects on health, including psychosexual function, circumcision does have side effects and complications including bleeding, postoperative and other infections, adhesions, fistulae and keloid.5
Pearly penile papules
Clinical features
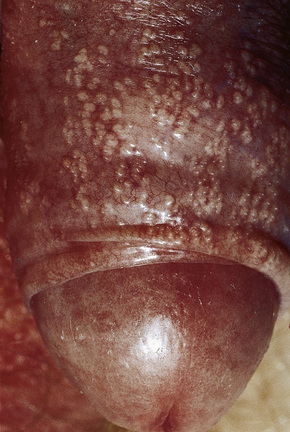
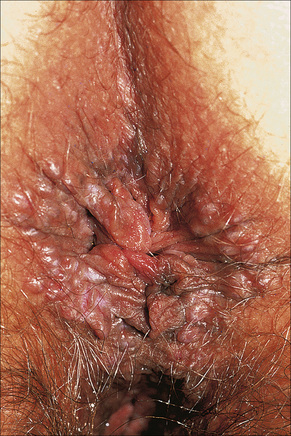
Pearly/pink penile papules1 may be found in 15–50% of men.2–5 They manifest as flesh-colored, smooth, rounded papules (1–3 mm) occurring predominantly around the coronal margin of the glans, rarely on the glans. Often there are rows or rings of papules (Fig. 12.13). Ectopic lesions, for example on the penile shaft, have been reported,6 including in children.7 They are frequently mistaken for warts and Tyson’s or ectopic sebaceous glands. The lesion is analogous to other acral angiofibromas such as adenoma sebaceum, subungual and periungual fibromas, fibrous papule of the nose, acquired acral angiofibroma and oral fibroma. These lesions may regress with circumcision and old age.8
Vestibular papillomatosis
Clinical features
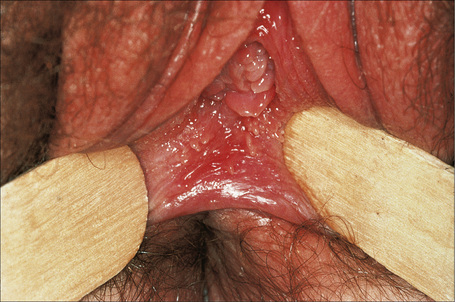
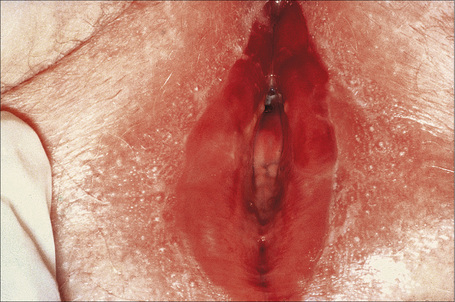
Vestibular papillomatosis is not due to human papillomavirus infection and is a common finding of no significance.1,2 It is asymptomatic and is the female equivalent of penile pearly papules (hirsutoid papillomas). Individual lesions are dome shaped or filiform and arise on a solitary base. They are found on the inner aspect of the labia minora and vestibule (Fig. 12.14).
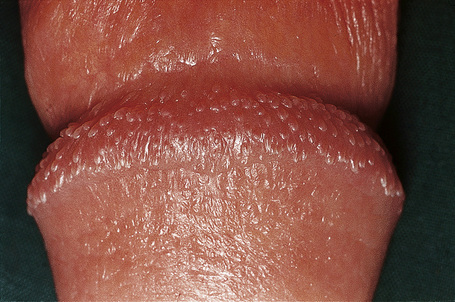
Sebaceous gland hyperplasia
Clinical features
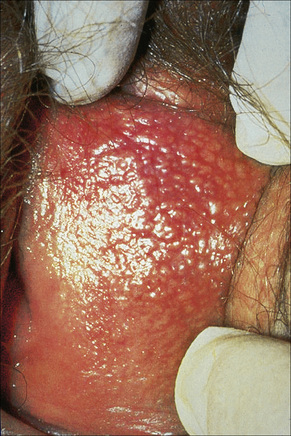
The sebaceous glands of the inner labia majora, the labia minora and clitoral prepuce do not usually have an associated hair follicle. The glands open directly onto the surface and may be very prominent and numerous at these sites. The yellow uniform papules (Fordyce spots) are often seen best if the skin is stretched (Fig. 12.15). Sebaceous gland hyperplasia may sometime be associated with pruritus and pain if they enlarge and the contents rupture into the dermis.
In the male, sebaceous gland prominence, Tyson’s glands, sebaceous hyperplasia and ectopic sebaceous glands (Fordyce’s condition) are all virtually synonymous and are common, normal variants of the skin of the scrotal sac and penile shaft (very rarely the glans) but may cause concern to patients even amounting to dysmorphophobia (Fig. 12.16).1,2
Inflammatory dermatoses
Intertrigo and balanoposthitis
Clinical features
These are non-specific terms.1 Intertrigo is the name given to any dermatosis occurring in skin folds: any scale is usually rapidly removed by frictional abrasion, and a degree of epithelial loss may result in erosion that renders the site especially susceptible to secondary infection, e.g., with Candida (see below).
Pathogenesis and histological features
Generally, dermatologists feel that balanitis, posthitis and balanoposthitis are probably more commonly due to inflammatory and precancerous dermatoses, while genitourinary physicians teach that most cases are due to infection, usually with Candida.1 However, Candida is a ready opportunist so its presence may not always indicate primary infection as the cause of the genital inflammation. Bacteria have increasingly been implicated in the etiology of the disease, particularly Staphylococcus aureus.2 In cases in which Streptococcus pyogenes is isolated it has been suggested that the disease may be sexually transmitted by penile–oral intercourse.3 Diabetes may be an important predisposing factor to Candida and other infective causes or complications of balanoposthitis.1
Non-specific balanoposthitis
Clinical experience and histological evidence indicate that non-specific balanoposthitis is a real entity.1–3 However, in practice it is a diagnosis of exclusion, because sexually transmitted diseases, eczematous dermatoses, psoriasis, Zoon’s balanitis, lichen planus, lichen sclerosus, mucous membrane pemphigoid and penile carcinoma in situ must be excluded. Non-specific balanoposthitis is a manifestation of a dysfunctional foreskin. Patients generally complain of pain during intercourse and may have variable signs, including eczematous, lichenoid and Zoonoid inflammation, and even scarring. Candida and other organisms can be identified, but they probably represent opportunistic infection. Diabetes should be excluded. In severe cases, medical treatments fail and the only cure is by circumcision. A prior clinical diagnosis may be reversed at this stage by foreskin histology showing lichen sclerosus, lichen planus or carcinoma in situ. However, experience shows that even where the most thorough histological examination of the excised prepuce is undertaken, nothing more than non-specific chronic inflammation is found.
Eczema
Clinical features
Seborrheic dermatitis is the commonest form of eczema affecting anogenital skin, followed by irritant contact eczema. Eczema, particularly seborrheic dermatitis, is more common than many other inflammatory dermatoses in uncircumcised males.1 Allergic contact eczema is rare at genital sites and occurs more typically in a perianal distribution. Involvement of genital skin with atopic eczema is very uncommon.
In infants, eczematous reactions are often seen in the napkin area. Most of these represent a primary irritant dermatitis. Seborrheic dermatitis and a psoriasiform napkin rash can also occur. Some but not all of the patients with the latter condition develop psoriasis later in life.2
Infantile gluteal granuloma
Clinical features
Infantile gluteal granuloma (papuloerosive dermatitis of Jacquet and Sevestre) is a rare condition that has been described mainly in the newborn and infants in the napkin area. It frequently develops in a background of an irritant napkin rash.1–4 A similar condition has been described in incontinent elderly women with lesions developing on the labia majora (Fig. 12.18).5–7 Oval or round papulonodular lesions present on the convex areas of the perineum, which are in direct contact with the napkin or incontinence pad. They tend to regress spontaneously over a few weeks and occasionally leave scars.
Lichen simplex chronicus
Clinical features
The clinical features of lichen simplex chronicus presenting on genital and anal skin are identical to those seen at other sites of the body. The mons pubis or labium majus, scrotum and perianal skin are the usual sites affected. Giant forms (of Pautrier) occur, e.g., on the scrotum, giving a pineapple appearance (Fig. 12.19).
Psoriasis
Clinical features
Flexural psoriasis is the most common pattern seen in the anogenital region, with extension into the genitocrural folds and natal cleft (Figs 12.20–12.23). There are often difficulties clinically in distinguishing between psoriasis and seborrheic dermatitis. Genital and flexural disease may reflect koebnerization and is relatively common.1 In the circumcised male the signs on the glans and distal penile shaft are similar to those of psoriasis at extragenital sites, whereas the appearances in the uncircumcised male are of balanoposthitis similar to flexural psoriasis.2
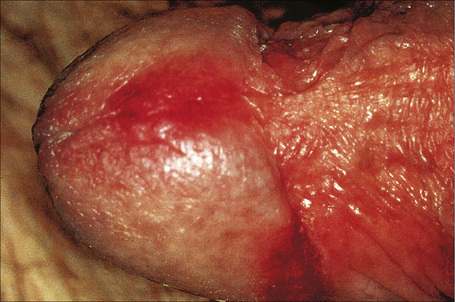
Fig. 12.21 Psoriasis: there are erythematous plaques on the glans penis.
By courtesy of the Institute of Dermatology, London, UK.
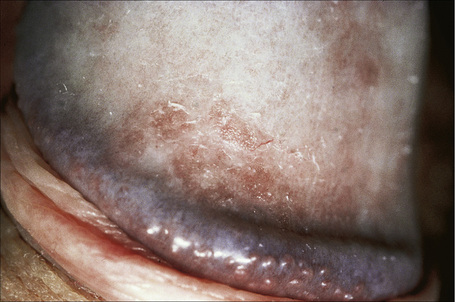
Fig. 12.22 Psoriasis: in this example a slight scale is apparent.
By courtesy of the Institute of Dermatology, London, UK.
Reiter’s syndrome
Clinical features
Reiter’s disease or syndrome is part of the same continuum as psoriasis in genetically predisposed individuals.1 Reiter’s syndrome is defined by the triad of arthritis, urethritis and conjunctivitis. The eponym is gradually losing favor in the face of more descriptive nomenclature and the term reactive arthritis is frequently used. It is precipitated by non-specific urethritis, bacillary or amebic dysentery and associated with HIV infection and the immunogenotype HLA B27.2–6 The classical triad may occur together or develop in sequence, and a range of other symptoms may also be present. It has a worldwide distribution. Reiter’s syndrome most commonly affects men 20–30 years of age; the male to female sex ratio is approximately 10:1.1 The syndrome is characterized by a relapsing course.4
The condition may follow an enteric or a urogenital infection.7,8 Shigella dysentery was the first associated enteric infection to be recognized and the causative organisms were either Shigella flexneri or S. dysenteriae.9 Recently, Salmonella, Yersinia and Campylobacter have been reported preceding Reiter’s syndrome.10–14
Sexually transmitted Reiter’s syndrome may occur with a nongonococcal or ‘non-specific’ urethritis.7Chlamydia trachomatis is isolated from the genitourinary tract in 40–60% of male cases; isolation is variable, however, and an indirect immunofluorescence test detects chlamydial infection in 90% of patients.15,16Mycoplasma infection and Streptococcus viridans have also been implicated.17–19 The condition has also been linked to the acquired immunodeficiency syndrome (AIDS).20–22 Rare associations include Cyclospora, Cryptosporidium, intravesical bacillus Calmette-Guérin (BCG) immunotherapy, Gardnerella vaginalis, hepatitis B immunization and systemic interferon-alpha (IFN-α) treatment.23–28
Reiter’s syndrome is more likely to occur in predisposed individuals. Human leukocyte antigen (HLA)-B27, which is thought to occur in up to 90% of patients, increases the risk of developing Reiter’s syndrome by 25 times; the disease is also more severe in HLA-B27-positive individuals.4,29 HLA-B27 in patients with Reiter’s syndrome correlates with ankylosing spondylitis.4 Reiter’s syndrome develops in 20% of HLA-B27-positive individuals after a specific infective episode.30 HLA-B27 is found in approximately 10% of the normal population. Recently, an association with HLA-B51 has been reported.31 Rarely, familial instances have been documented.30 Therefore it appears that the disease is triggered in genetically predisposed individuals by an unknown mechanism precipitated by infection.
Bilateral mucopurulent conjunctivitis is the usual form of eye involvement occurring in up to 35% of patients, but occasionally iritis, iridocyclitis, keratitis or blindness occurs.32
Weight-bearing joints and the larger ones (knees, ankles, feet and wrists) are involved by the arthritis, often together with sacroiliitis. Radiological changes include osteoporosis, erosions and loss of joint space, with multiple joints usually affected.4,30 Periostitis often affects the metatarsals, the phalanges of the feet and the tarsal bones; occasionally, ankylosis develops in the small bones of the hands and feet. Ankylosing spondylitis, which is an important manifestation, correlates with a high erythrocyte sedimentation rate (ESR).30
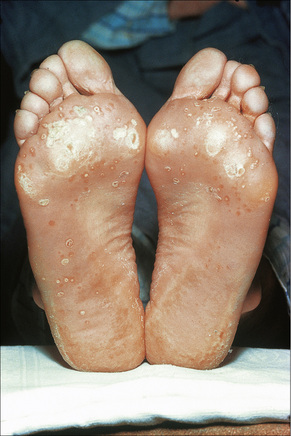
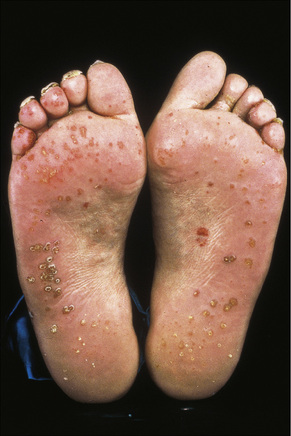
Skin lesions in Reiter’s syndrome may be similar to those of psoriasis. Cutaneous manifestations include hyperkeratotic cobblestone lesions on the palms and soles and occasionally affecting the trunk and extremities (Fig. 12.24). The lesions initially present as erythematous macules; over the course of several days these become hyperkeratotic waxy papules, with an erythematous halo covered by dry hyperkeratotic material. The papules are numerous and eventually coalesce to form thickened horny plaques. Pustular lesions of the palms and soles may also be evident (keratoderma blenorrhagicum) (Fig. 12.25).
Circinate balanitis, presenting as a moist superficial erosion, 2–4 mm across, may affect the glans penis and meatus (Figs 12.26–12.28). Superficial ulceration of the oral mucosa may also occur, together with reddening and a granular appearance of the surrounding mucous membrane.4,5
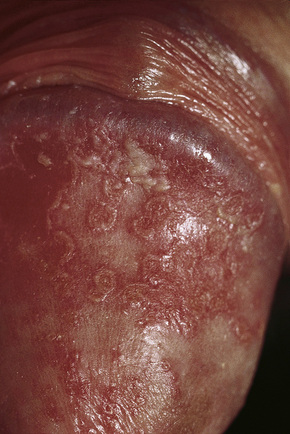
Fig. 12.26 Circinate balanitis. Glans penis. Psoriasiform lesions.
From Bunker C: Male Genital Skin Disease. Saunders Ltd./Elsevier 2004.
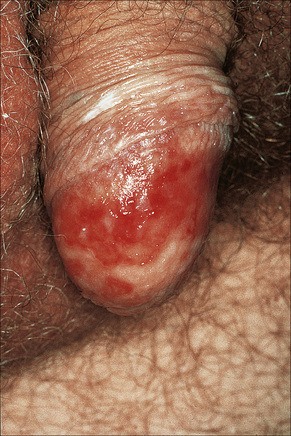
Fig. 12.27 Reiter’s syndrome: there are multiple erosions on the glans penis.
By courtesy of The Institute of Dermatology, London, UK.
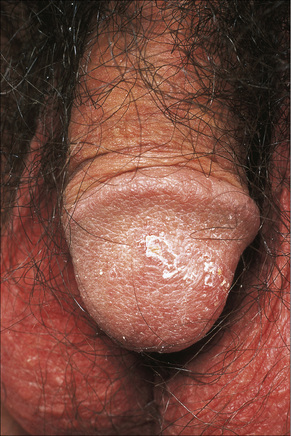
Fig. 12.28 Reiter’s syndrome: in this patient there are scaly lesions on the glans penis.
By courtesy of the Institute of Dermatology, London, UK.
Stomatitis and nail dystrophy (indistinguishable from that of psoriasis) may be additional features.4 Weight loss is common.20 Aortic incompetence is an important late complication and IgA nephropathy has been described in a number of patients.4,33
As stated before, Reiter’s syndrome is very rarely reported in females, and the majority of cases recognized are in males.34–38 Vulvitis has been described in a small number of case reports.34–38 and in one case cervical lesions were seen.37 The vulval lesions are erythematous and may be ulcerative, eroded or scaly, and often resemble mucocutaneous candidiasis. The cervical lesions were recorded as white papules.37 There may be accompanying oral lesions.
Reiter’s syndrome may resolve spontaneously, but more often it is characterized by chronicity and recurrences.4 Rarely, it may prove fatal.4 Causes of death include aortic incompetence, atrioventricular block, terminal cachexia, systemic amyloidosis and iatrogenic effects.39
Histological features
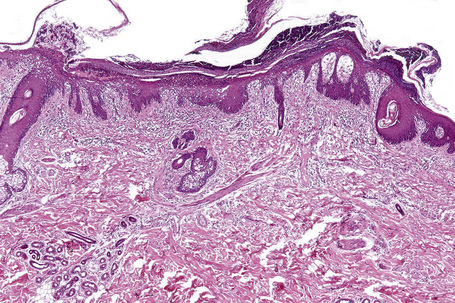
Essentially, just as the skin lesions of Reiter’s syndrome have psoriasiform morphology (Fig. 12.29), they have the same histopathology and ultrastructure as pustular psoriasis.40
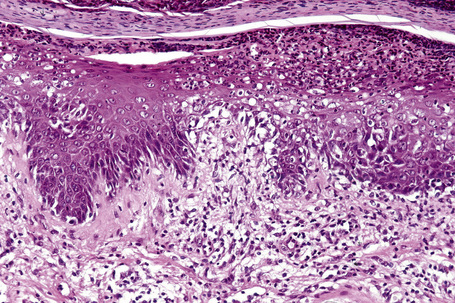
The epidermis is acanthotic with elongation and hypertrophy of the epidermal ridges and parakeratosis. The suprapapillary plates are thinned and there is infiltration of the epidermis by neutrophils, associated with vacuolation of superficial keratinocytes, together with the formation of spongiform pustules and microabscesses (Fig. 12.30). The inflammation extends into the adjacent underlying dermis where it is predominantly mononuclear. The histology is essentially identical to that seen in pustular psoriasis.2 Therefore, close clinicopathological correlation is critical to establish a diagnosis. Occasional biopsies from typical lesions of patients with Reiter’s syndrome may disclose an underlying leukocytoclastic vasculitis.41
A small number of patients presenting with skin lesions that histologically showed sterile neutrophilic folliculitis with perifollicular vasculopathy have been documented.42 The authors suggested that this histological pattern may be a marker of systemic disease. Associations may include Reiter’s syndrome, inflammatory bowel disease, Behçet’s disease, hepatitis B infection, scrofuloderma, connective tissue diseases and hematological dyscrasias. Patients present with systemic symptoms and variable skin lesions including folliculitis, vasculitis, acneiform eruptions, vesiculopustules and erythema nodosum-like features.
Early joint lesions are characterized by a neutrophil polymorph inflammatory cell infiltrate with little if any synovial changes. Older lesions show features suggestive of rheumatoid arthritis, including lymphoid aggregates, a perivascular chronic inflammatory cell infiltrate and synovial hyperplasia.30
Genital lichen planus
Clinical features
Anogenital lesions may be found in up to 40% of patients with generalized disease. In some, however, the disease is restricted to the lower genital tract and/or the perianal region, and in these instances the diagnosis may be difficult to establish.1,2 Lichen planus (LP) manifests the Koebner phenomenon which may partly explain the orogenital predilection.3 Genital lichen planus in children is exceptional.4
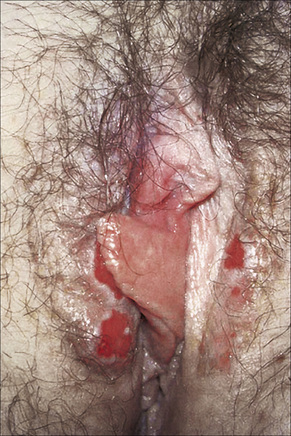
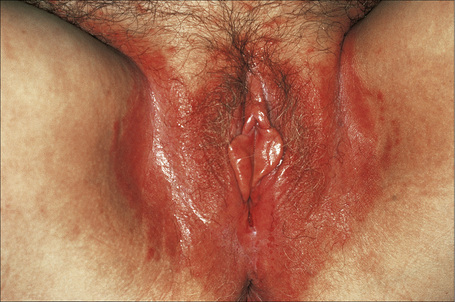
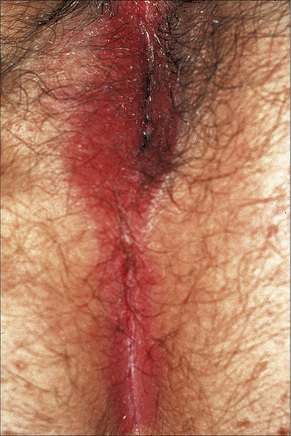
The lesions are typical, violaceous or white patches or areas of erythema and erosions. Wickham’s striae (frequently seen in oral involvement), although sometimes visible, are less often found on anogenital skin (Fig. 12.31). The erosive form of LP is commoner at anogenital sites and can lead to scarring and distortion of the architecture (Figs 12.32–12.34). The vulval vestibule and vagina and cervix may also be involved and sometimes the vagina and or the cervix are affected alone.5–7 Perianal disease can lead to deep, painful fissuring and it is often the hypertrophic variant that involves this site. Women with oral lichen planus often have genital disease and they frequently have asymptomatic lesions.8,9
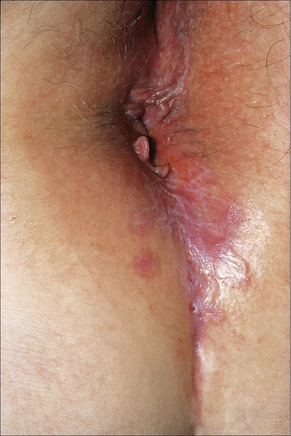
Fig. 12.31 Lichen planus: perineal lesions showing conspicuous striae.
By courtesy of the Institute of Dermatology, London, UK.
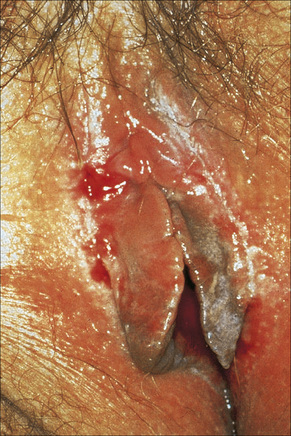
Fig. 12.32 Erosive lichen planus: bilateral erosions are present.
By courtesy of the Institute of Dermatology, London, UK.
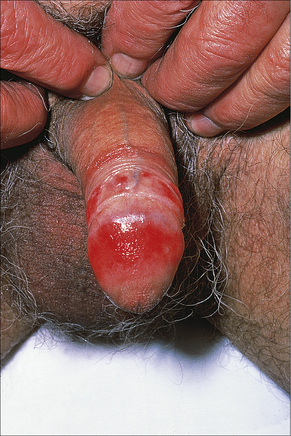
Fig. 12.33 Erosive lichen planus: there is extensive erosion of the glans penis.
By courtesy of the Institute of Dermatology, London, UK.
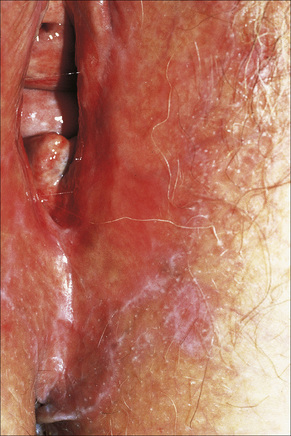
Fig. 12.34 Vulval lichen planus: reticulated lesions of lichen planus extending into the perineum.
By courtesy of the Institute of Dermatology, London, UK.
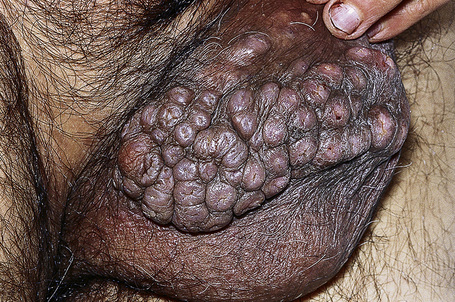
The clinical variants of lichen planus affecting the anogenital skin are usually squamopapular with areas of erosion, hypertrophic and hyperpigmented flexural disease (Figs 12.35–12.39). In the male genital, LP can present as phimosis.10,11 Adhesions are seen in the uncircumcised male, both transcoronal and subcoronal.
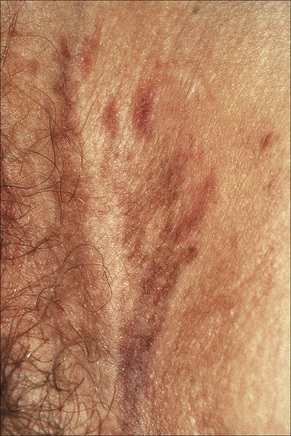
Fig. 12.35 Vulval lichen planus: in this example of resolving disease there are linear hyperpigmented lesions.
By courtesy of the Institute of Dermatology, London, UK.
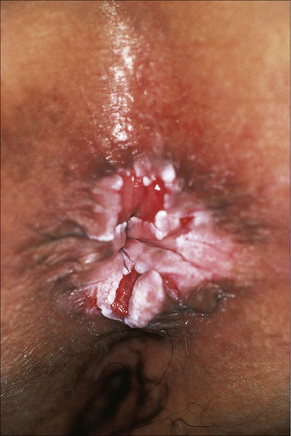
Fig. 12.36 Perineal lichen planus: typical papules with Wickham’s striae are present.
By courtesy of the Institute of Dermatology, London, UK.
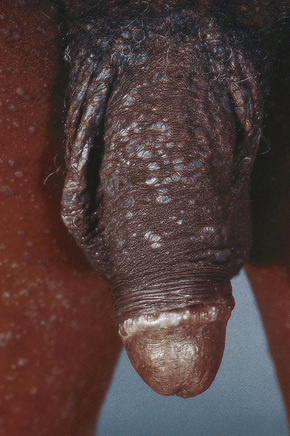
Fig. 12.38 Penile lichen planus: there is involvement of the shaft and glans.
From Bunker C: Male Genital Skin Disease. Saunders Ltd./Elsevier 2004.
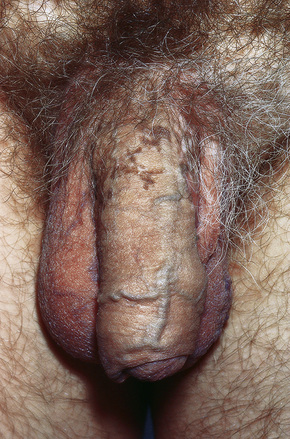
Fig. 12.39 Penile lichen planus: the proximal shaft shows post inflammatory hyperpigmentation.
From Bunker C: Male Genital Skin Disease. Saunders Ltd./Elsevier 2004.
There is also an unusual variant of erosive LP in women that involves the oral gingivae, vulval vestibule and vagina, known as the vulvovaginal-gingival syndrome (Figs 12.40–12.42).12,13 This can lead to severe vulval and vaginal scarring with vaginal adhesions, constriction bands and, in some cases, complete stenosis.14 A male equivalent to the vulvo-vaginal syndrome of Hewitt with chronic erosive gingival and genital lesions (genito-gingival syndrome) has been described.15,16 Patients with genital lesions may have anal, oral, aural, conjunctival and esophageal involvement.17–24 Those patients with predominantly mucosal disease clinically mimic mucous membrane pemphigoid but immunofluorescence studies are invariably negative. A case of paraneoplastic lichen planus with orogenital involvement and cicatrizing conjunctivitis in association with thymoma has been described.25
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree