Patsy M. Brannon, PhD, RD, Patrick Tso, PhD and Ronald J. Jandacek, PhD The central challenge for the digestion and absorption of dietary lipids (defined in Chapter 6) is their insolubility in the aqueous environments that characterize the gastrointestinal lumen and enterocyte (small intestinal absorptive cell). To meet this challenge, coordinated and complex processes involving emulsification, digestion, micellar solubilization, uptake, intracellular transport, and packaging into lipoproteins for transport must occur. These processes begin in the stomach, continue in the lumen of the small intestine, and conclude in the enterocyte. Digestion of lipids depends upon actions of amphipathic enzymes, other proteins, and bile acids (bile salts) that act at the lipid–aqueous interface. Although a variety of lipids are consumed in the diet, nonpolar triacylglycerols represent the largest component (90% or more) and provide the polyunsaturated/essential fatty acids (discussed in Chapter 18). These triacylglycerols contain predominantly long-chain fatty acids (chain lengths of 14 to 20 carbons) esterified to the glycerol backbone. According to the 2000-2006 National Health and Nutrition Examination Survey (NHANES), the average daily consumption of fat (triacylglycerols) by American adults is approximately 65 g for women and 95 g for men (34% of the caloric intake). Other dietary lipids include cholesterol; cholesteryl esters; plant sterols; plant sterol esters; the fat-soluble vitamins A, D, E, and K (discussed in Chapters 28 through 31); carotenoids; and phospholipids. Daily dietary intake of sterols includes 275 mg of cholesterol (NHANES 2000-2006), 100 to 400 mg of plant sterols and other noncholesterol sterols, and 1 to 2 g of phospholipids. Secretion of endogenous biliary lipids into the intestine present far more additional cholesterol (1 g) and phospholipids (10 to 20 g) to the intestine over the course of a day than are consumed in the diet. The fat-soluble vitamins and carotenoids are consumed in vastly smaller amounts, typically in milligram or microgram amounts. Hydrolysis of triacylglycerols, cholesteryl esters, and phospholipids is absolutely required for their absorption from the intestinal lumen into the enterocyte. Multiple lines of evidence conclusively demonstrate this requirement: (1) the failure to absorb silver-containing oil droplets in electron micrograph studies (Cardell et al., 1967); (2) the nonabsorbability of nonhydrolyzable polyoleate esters (Mattson and Volpenhein, 1972); and (3) the reduction of fat absorption by the partial inhibition of the primary hydrolytic enzymes, gastric and pancreatic lipases; by orlistat (tetrahydrolipstatin, Xenical) in humans (Carrière et al., 2001); or by the combined knockout of pancreatic hydrolytic enzymes (lipase and carboxylester lipases) in mice (Gilham et al., 2007). Thus the first critical process in lipid digestion and absorption is the hydrolysis of the lipid esters. Digestion of triacylglycerols in humans begins in the stomach with the action of the hydrolytic enzyme, gastric lipase, which is secreted by the gastric mucosa. This lipase is most active in an acidic environment. It preferentially hydrolyzes the fatty acid at the sn-3 position of the triacylglycerol molecule, regardless of the fatty acid esterified to this position (Aloulou and Carrière, 2008), but it can also hydrolyze fatty acids at the sn-1 position (Whitcomb and Lowe, 2007). Although the optimal pH of gastric lipase is around 4, the enzyme is still active at pH 6 to 6.5 and probably continues to hydrolyze triacylglycerol in the upper duodenum where the pH is between 6 and 7. In cases of pancreatic insufficiency, gastric lipase may compensate at least partially for the loss of the pancreatic hydrolytic lipases. Overall, the net digestion of fat in the stomach is limited (less than 10% to 25%), but it contributes to the subsequent digestion of fat in the lumen. Gastric lipase plays an important role in the digestion of fat in human milk and formula by infants, who have low levels of the major hydrolytic enzyme, pancreatic lipase, until weaning (Lindquist and Hernell, 2010; Whitcomb and Lowe, 2007). As discussed later, pancreatic lipase-related protein 2 and carboxylester lipase also contribute to the digestion of fat by the infant. Pancreatic lipase, which has a pH optimum around pH 8, digests most of the triacylglycerol that enters the small intestine (Whitcomb and Lowe, 2007), acting along with the contributing activities of carboxylester lipase (Gilham et al., 2007) and possibly pancreatic lipase-related peptide 2 (Figure 10-1). Pancreatic lipase, which is secreted in its active form, works at the lipid−aqueous interface of the emulsified lipid particles and mainly hydrolyzes the sn-1 and sn-3 positions of triacylglycerol molecules to release 2-monoacylglycerol and free fatty acids. However, the bile acids at the lipid−aqueous interface of the emulsified lipid particles inhibit the action of pancreatic lipase unless colipase is present. Colipase is also secreted by the pancreas as the inactive procolipase, which is activated by trypsin-mediated proteolytic cleavage in the small intestine. The binding of colipase to the lipid–aqueous interface allows the binding and anchoring of lipase to the interface. This binding in a 1:1 molar ratio also results in important conformational changes in lipase that expose its active site and enable its hydrolytic action. Colipase deficiency in humans and mice results in fat malabsorption. In contrast, pancreatic lipase-related protein 2 has broad activity and hydrolyzes triacylglycerols, phospholipids, and galactolipids, depending on the species (Whitcomb and Lowe, 2007) and is neither stimulated by colipase nor inhibited by bile acids. Its action appears important for triacylglycerol digestion in the newborn when its secretion is high. In knockout mice lacking pancreatic lipase-related protein 2, fat malabsorption is present in pups until weaning when pancreatic lipase secretion increases (Lindquist and Hernell, 2010). Digestion of phospholipids occurs in the small intestine through the hydrolytic action of pancreatic phospholipase A2, which acts at the lipid interface of micelles. Pancreatic phospholipase A2 acts on both the dietary and the endogenous phospholipids. The most abundant of these phospholipids is phosphatidylcholine. The pancreas secretes an inactive prophospholipase A2, which is then activated by trypsin-mediated cleavage in the lumen. Phospholipase A2 hydrolyzes the fatty acid from the sn-2 position of phosphatidylcholine to yield a fatty acid and lysophosphatidylcholine (Whitcomb and Lowe, 2007). Some minor contribution may also come from the intestinal mucosal intrinsic membrane enzyme known as retinyl ester hydrolase or phospholipase B, which has both phospholipase and retinyl ester hydrolase activities. The relative contributions of lipid digesting enzymes is not fully understood but is known to depend on the stage of development and the relative availability of the various lipases. As stated earlier, gastric lipase and pancreatic lipase-related peptide 2 appear to be able to digest completely the dietary lipid in breast milk or formula, because pancreatic lipase is very low in the newborn. Carboxylester lipase is present in secreted breast milk and also contributes to fat digestion in the newborn (Whitcomb and Lowe, 2007). Studies in knockout mice lacking one or more lipases also reveal the redundancy and compensation of the lipases in lipid digestion. Deficiency of pancreatic lipase does not affect triacylglycerol absorption in mice fed a low-fat diet but reduces it in those fed a high-fat diet and also reduces cholesterol absorption (Gilham et al., 2007). Loss of carboxylester lipase does not affect retinyl ester absorption, although it does reduce cholesteryl ester absorption. Deficiency of phospholipase A2 does not affect phospholipid absorption. However, deficiency of both pancreatic lipase and carboxylester lipase reduces both triacylglycerol and retinyl palmitate digestion and absorption (Gilham et al., 2007). Similarly during pancreatic insufficiency in humans, gastric lipase contributes to the partial digestion of dietary lipid. Clearly, the overlapping and complementary functions of the lipid digesting enzymes allow for redundancy and compensation in lipid digestion. Micellar solubilization of the digestion products, predominantly monoacylglycerols, fatty acids, lysophosphatidylcholine, and cholesterol, is essential to their uptake by enterocytes. Micellar solubilization enables the lipid digestion products to cross the barrier presented by the unstirred water layer surrounding the epithelial surface of the small intestine. The unstirred water layer varies in thickness depending on how vigorously the intestinal contents are mixed (Figure 10-2). Without micelle formation, the low solubility of lipid digestion products in water would limit their ability to cross this unstirred layer. Micelles form when the bile acid concentration is at or above the critical micellar concentration (~1 to 2 mmol/L, depending on the specific bile acid). In the human small intestinal lumen, the bile acid concentration is almost always above the critical micellar concentration, such that bile acid micelles form (Hofmann, 2009). Once incorporated into the mixed micelles (containing bile acids and other lipid moieties), lipid digestion products readily cross the unstirred water layer, resulting in a 100- to 1,000-fold increase in the aqueous concentrations of fatty acids, monoacylglycerols, cholesterol, and lysophosphatidylcholine near the epithelial absorptive surface. Thus micellar solubilization provides an efficient mechanism for diffusion of lipid digestion products across the unstirred water layer, facilitating the subsequent uptake of these lipid molecules by the enterocytes. The micellar phase, however, is not homogeneous. In addition to the large disc-like mixed bile acid micelles that are more or less saturated with lipids, lipid crystalline or unilamellar vesicles exist and are composed of mixed lipids saturated with bile acids (Carey et al., 1983). These vesicles may play an important role in the uptake of lipid digestion products in patients with low intraluminal bile acid concentrations (Mansbach et al., 1980) or bile fistulae (Porter et al., 1971). Identification of specific transporters for fatty acids has been controversial. Although fatty acid transport protein 4 was initially suggested to play a role in the absorption of fatty acids, current evidence suggests that it does not (Shim et al., 2009). Instead, fatty acid transport protein 4 is localized to the endoplasmic reticulum (ER) membrane and plays a role in the intracellular processing of fatty acids (Mansbach and Gorelick, 2007). Emerging instead is the role of the fatty acid translocase, CD36 (Figure 10-3), in the absorption of fatty acids from the proximal, but not distal, intestine (Nassir et al., 2007). CD36, a glycoprotein, is a member of the type B scavenger receptor family that translocates long-chain fatty acids across the apical membrane of the enterocyte, but the precise mechanism of this transfer is not understood. CD36 may act in concert with other membrane and intracellular proteins to effect this transfer, although the nature and identity of these other proteins is not known. Another transport protein, NPC1L1, plays an essential role in cholesterol and phytosterol uptake (Hui et al., 2008; Betters and Yu, 2010; see Figure 10-3). The highest expression of NPC1L1 in the proximal small intestine colocalizes with the site of maximal cholesterol absorption. Both NPC1L1 and cholesterol absorption are minimal in the distal small intestine. Deficiency of NPC1L1 in knockout mice reduced cholesterol absorption by 70% (Altmann et al., 2004). Further, NPC1L1 is the target of ezetimibe, a potent inhibitor of cholesterol and plant sterol absorption (Betters and Yu, 2010). This transporter protein cycles between the apical surface and intracellular compartments of the enterocyte in a mechanism consistent with cholesterol-regulated, clathrin-mediated endocytosis. Details remain to be elucidated, but NPC1L1 may act as an extracellular free cholesterol receptor, recruiting extracellular cholesterol to its N-terminal until the cholesterol content in this microdomain of the apical membrane reaches the threshold necessary for endocytosis and targeting to intracellular compartments (Betters and Yu, 2010). At least two other proteins, scavenger receptor B1 (SR-B1) and CD36 may also play roles in cholesterol absorption, particularly in the proximal intestine (Hui et al., 2008). Like NPC1L1, SR-B1 is inhibited by ezetimibe. The relative contributions of NPC1L1, SR-B1, and CD36 and their potential interactions in cholesterol absorption are not fully understood. Complicating assessment of the net absorption of cholesterol, however, is the efflux of absorbed cholesterol from the enterocyte through the ATP-binding cassette proteins (ABCG5 and ABCG8) that are located on the enterocyte’s apical membranes (Hui et al., 2008; see Figure 10-3). Their expression is highest in the proximal and midsections of the small intestine; they limit net cholesterol absorption by transporting cholesterol back into the lumen of the intestine. Furthermore, these ABC transporters efficiently transport plant sterols, which differ from cholesterol only by a methyl or ethyl group in the side chain, out of the enterocyte. Thus only a small percentage (≈1%) of ingested noncholesterol sterols are absorbed and retained in the body, whereas 50% to 60% of dietary cholesterol is absorbed and retained. This tremendous ability of the small intestine to discriminate against the plant sterols is lost in patients with β-sitosterolemia, a rare autosomal recessive disorder characterized by hyperabsorption of all sterols. Mutations in the ABCG5 or ABCG8 genes cause β-sitosterolemia (Lu et al., 2001). Many of these mutations seem to prevent formation of stable heterodimers and to impair trafficking of the sterol transporter to the apical surface of the cells (Graf et al., 2004), thus reducing the efflux of plant sterols back to the lumen of the intestine.
Digestion and Absorption of Lipids
Luminal Digestion of Lipids
Gastric Digestion of Triacylglycerols
Luminal Small Intestinal Digestion of Triacylglycerols
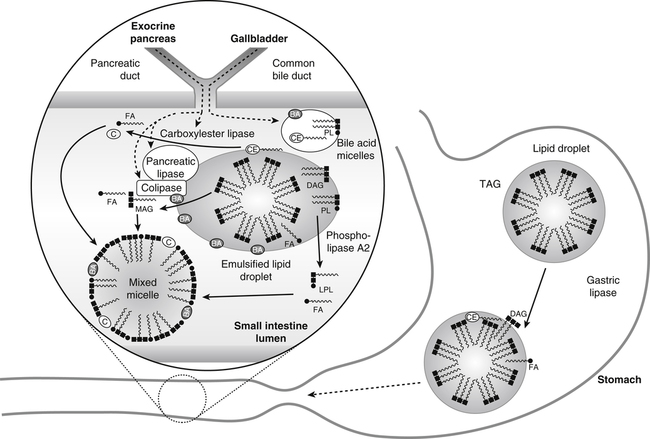
Gastric lipase hydrolyzes triacylglycerol (TAG) in large lipid droplets to diacylglycerol (DAG) and fatty acids (FA) in the stomach while the mixing and grinding of the stomach emulsifies the lipid. The emulsified lipid droplets enter the small intestine where they stimulate release of cholecystokinin. This in turn causes the gallbladder to release bile acids (BA), which are largely in the form of micelles with endogenous cholesteryl ester (CE) and phospholipid (PL). These bile acids partition into the emulsified lipid droplet. The exocrine pancreas secretes pancreatic lipase and its required cofactor colipase, phospholipase A2, and carboxylester lipase. After activation by the action of trypsin, pancreatic lipase and colipase bind together to the surface of the BA-covered lipid emulsion, inducing a conformational change in phospholipase to enable the hydrolysis of TAG and DAG to monoacylglycerol (MAG) and FA. Phospholipase A2 hydrolyzes PL to lysophospholipids (LPL) and FA. Carboxylester lipase hydrolyzes CE to cholesterol (C) and FA. The lipid digestion products partition into the mixed micelles. Note that lipid droplets and micelles are not drawn to scale. Lipid droplets are about 500 nm in diameter, whereas micelles are about 5 nm in diameter.
Luminal Small Intestinal Digestion of Phospholipids
Relative Contributions of Lipid Digesting Enzymes
Uptake of Lipid Digestion Products by the Enterocytes
Micellar Solubilization of Lipid Digestion Products
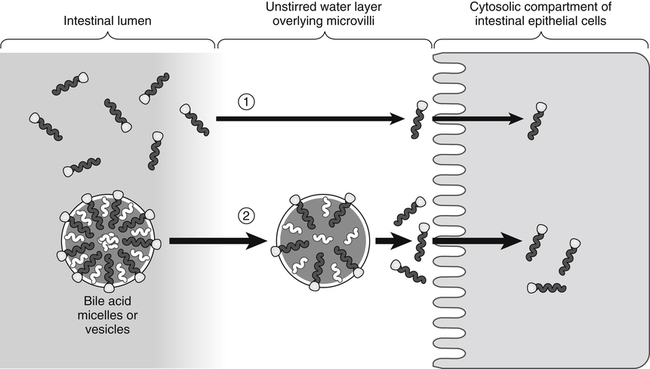
In the absence of bile acids (1), only a limited number of lipid molecules diffuse through the unstirred water layer to be taken up across the brush border membrane of the enterocytes. In the presence of bile acids (2), more lipid molecules can be delivered to the brush border membrane by bile acid micelles.
Absorption of Products of Lipid Digestion by Enterocytes
Carrier-Mediated Uptake of Fatty Acids
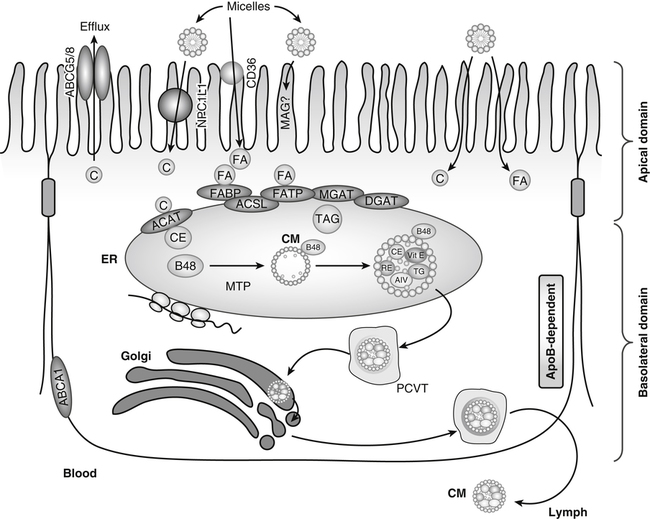
Products of lipid hydrolysis are solubilized in micelles and presented to the apical membranes of enterocytes. This membrane harbors several transport proteins that participate in the uptake of various types of lipids. Niemann-Pick C1 like 1 (NPC1L1) is a protein involved in cholesterol uptake. CD36 participates in fatty acid (FA) absorption. In addition, passive diffusion of lipid digestion products also occurs. In the cytosol, FA-binding protein (FABP) transports FAs to the ER where fatty acid transport protein-4 (FATP) or acyl-CoA synthetase long-chain family members (ACSL) activates them to acyl-CoAs for subsequent esterification. Acyl-CoA:cholesterol acyltransferase (ACAT), monoacylglycerol transferase (MGAT), and diacylglycerol acyltransferase (DGAT) are found in the membranes of the endoplasmic reticulum (ER), where they facilitate the esterification of cholesterol (C), monoacylglycerols (MAG), and diacylglycerols (DAG), respectively. These esterified products, triacylglycerols (TAG) and cholesteryl esters (CE), are incorporated into apolipoprotein (apo) B-48−containing chylomicrons (CM) in a microsomal triacylglycerol transport protein (MTP)-dependent manner. The newly synthesized prechylomicrons are transported in specialized vesicles, the prechylomicron transport vesicles (PCTV), to the Golgi apparatus for further processing and for secretion. In addition, enterocytes express ATP-binding cassette (ABC) transporter A1 on the basolateral membrane to facilitate the efflux of cholesterol and plant sterols. (Adapted from Iqbal, J., & Hussain, M. M. [2009]. Intestinal lipid absorption. American Journal of Physiology Endocrinology and Metabolism, 296, E1183–E1194.)
Carrier-Mediated Uptake of Cholesterol
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Digestion and Absorption of Lipids
