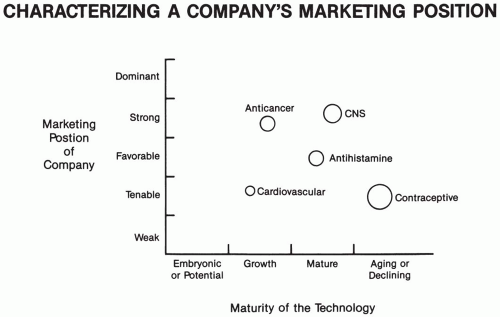
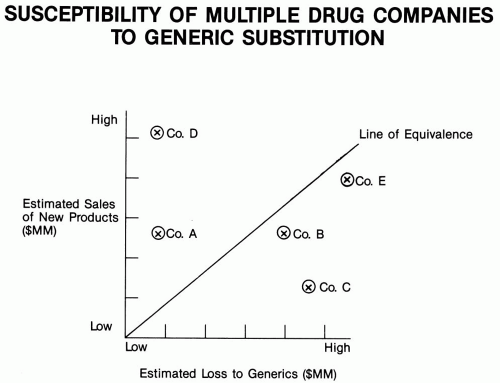
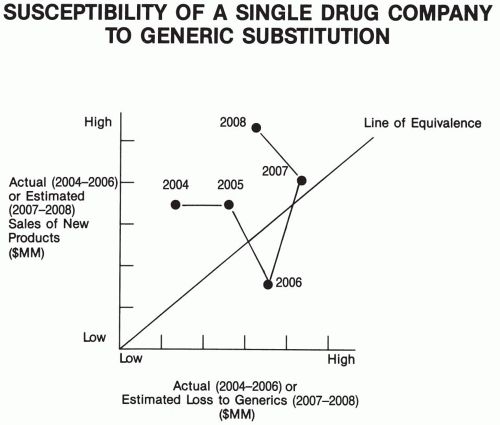
Background
Within any company, there are many philosophical and other differences concerning the methods that are the most efficient, most ethical, most practical, and most cost-effective for developing drugs. It is a happy but rare occasion when there is total agreement among all managers about how to develop even a single drug.
This issue becomes more complex when a company is simultaneously developing a particular drug in two or more countries. This is especially relevant if the company is not run as a centralized “dictatorship,” and each company site running a development program for the drug has a voice in the decision-making process. There are a number of issues that are generally addressed differently in various countries, and these differences often place strains on any unified development plan. Differences
within a company are usually a reflection of the types of differences that also exist between companies.
Although many of these differences are discussed throughout the book, some overall areas where differences exist are reviewed in this chapter.
Company Culture
A company’s culture is a living and dynamic process that often changes when new leaders are promoted from within, or brought into a company from elsewhere to help move it in a certain direction. Such changes may indicate a change in policy to employees. Leaders impose their ideas and style on how they want the company to operate and to be viewed by both internal staff and the external world. Companies vary greatly along the continuum of multiple cultural scales and factors. These scales include paternalism, benefits, personality and values of employees hired and promoted, ability for certain staff to spend time outside the company participating in activities of professional societies, time allowed for certain staff to spend on activities of the trade associations the company is a member of, and so forth.
While a company’s officers generally choose the way they want the corporate culture to be developed and maintained, they may wish to take periodic soundings to assess the culture. This practice can help ensure that others see the company the same way that they do, and can be accomplished through many techniques varying from informal conversations to formal evaluations or surveys conducted by a vendor hired for this purpose. There is, unfortunately, often one company, culture, or image that is widely promulgated and appears in the annual reports and newsletters in some companies, but this does not always match the culture that is supported and actually is in place. One example is that many companies speak about innovation, or emphasize the importance of patients they are trying to help, but in reality scientists are not allowed to fail more than once in trying to learn about an area they are researching or to test a hypothesis, and drugs that would truly help many patients are often not developed if the estimated sales projections do not meet the company’s hurdle rates. Moreover, these drugs are often not licensed to other companies that would want to develop them.
Positive cultural attributes, behavioral adherence to the purported cultural values, as well as a vibrant institutional memory, can help build staff dedication and loyalty by motivating employees and can be used as a significant tool for recruiting new employees. Investors and the stock market press value a dedicated and loyal workforce.
The culture of a pharmaceutical company influences the approaches used in drug development. A highly conservative approach to drug development is where each major question that could be raised by a regulatory agency has been studied and discussed. A less conservative approach could be to conduct a minimal number of studies and to assemble a lean New Drug Application in terms of quantity of data. Proponents of the lean approach hope that various potential issues about the drug are not raised and that the quantity of data submitted will be sufficient to obtain regulatory approval. Other aspects of the impact of a company’s culture on drug development relate to whether a company is prone to challenge the Food and Drug Administration (
FDA) requests for additional data or whether the company accepts
FDA requests without comment. Some companies attempt to achieve a close “alliance” with the
FDA on a drug’s development from the time the initial plan is formulated through each stage of its development.
Organizational Levels at Which to View Culture
Culture may be viewed on at least three separate levels. The first level is
the overall company level, where one may focus on aspects that distinguish each company. These include the internal company environments, traditions, values, image, and reputation, plus the personalities and style of the most senior leaders. Cultures might differ in the relative influence of marketing and research and development (
R and D) in major decisions made about identifying therapeutic areas to research and specific drugs to develop and prioritize. Either marketing or
R and D might have a major role in establishing the company’s goals and objectives.
The second level is
that of the entire function (e.g.,
R and D, marketing, or production). This level of culture depends on the same aspects as the corporate level plus the nature of the present staff and the most senior manager or leader and the characteristics of the specific function.
The third level relates to the specific people with whom each worker directly interacts in his or her daily activities. This level of culture depends on all of the above-noted factors plus the specific attributes and environment of the person or group involved and those with whom they interact.
Knowledge of a company’s culture is essential. It allows people to know (a) who to go to when you want things done, (b) where the real power lies, (c) what types of comments or constructive criticisms can be made, and (d) how to build a consensus in achieving one’s objectives. It allows people to function more effectively in their various roles.