Diagnostic Laparoscopy
Kevin C. Conlon
Paul F. Ridgway
Introduction
Evolution of the Role of Laparoscopy
Laparoscopy represents a disruptive technology, where the technology occasions change in indications for treatment, not just the method of treatment. Its promise of reduced pain scores following the laparoscopic resectional surgery, shorter postoperative ileus, and shorter hospital stay, as well as better cosmesis has promoted its usage in many general surgical and gynecologic procedures.
Laparoscopy further represents a shift in management attitudes toward rehabilitation and discharge planning. Even in societies where length of stay includes a period of convalescence, laparoscopy represents a minimally invasive strategy that facilitates enhanced recovery programs and early discharge. It is noteworthy, however, with integrated clinical pathways, that hospital stay following open colonic resection can be reduced to 2 days, indicating that the differences shown in other studies may not be so clear-cut.
Much was written of endoscopic surgery about 100 years ago; many specialties have dabbled in various techniques that have accessed body cavities by rudimentary rigid instrumentation. Laparoscopic examination of the abdominal cavity in humans was first described in 1910 by Jacobaeus, a Swedish physician. Two years later, he published a 97-patient series performed between 1910 and 1912 at Stockholm’s Community Hospital. However, it was not until the routine diagnostic use of laparoscopy was fostered by gynecology in the early 1970s that laparoscopy became more commonplace. Endometriosis, infertility, and ovarian cystic disease seem eminently suitable for laparoscopic evaluation. General surgeons were rather late adopters with the routine use of laparoscopy coming in the 1990s. The benefits quickly became apparent in the treatment of conditions such as cholecystectomy and appendectomy. Once the initial learning curve issues (Sweeney et al.) had been addressed, laparoscopy has replaced laparotomy as a default pathway for the operative management of many general surgical pathologies.
Rationale for Diagnostic Laparoscopy
The early authors who described techniques for induction of the pneumoperitoneum were almost universal gynecologists like Dr Hasson (whose name is frequently used synonymously with the open technique). Many early techniques thus centered on the pelvic organs. At a time when cross-sectional imaging was significantly less sophisticated than today, it seems a natural evolution that laparoscopy would have benefit in evaluation in many general surgical benign and malignant pathologies.
Diagnostic laparoscopy is safe, available, and may be applied in the contemporary management of a range of conditions including the acute and elective abdomen and pelvis. Its current role is as a replacement for exploratory laparotomy, particularly in oncological staging.
It is noteworthy that introduction of surgical technologies is not submitted to the rigorous study that new medicines require. This is particularly important where the use of minimal access techniques in the surgical diagnosis of cancer should be beneficial in terms of the oncological effect on the tumor compared with conventional surgery or simple imaging. Murthy et al. in 1989 demonstrated that surgical injury itself promotes tumor growth and others showed that reducing peritoneal trauma resulted in a decrease in tumor cell implantation. There
are many theories, best summarized by Ziprin’s work in 2002, but the message is the same; conditions specific to the laparoscopic surgical environment promote implantation and a more aggressive invasive capability for shed tumor cells. Thus, therapeutic use of laparoscopies in the resection of intra-abdominal malignancies deserves separate analysis, and although its introduction was fraught, it has lessons for us all.
are many theories, best summarized by Ziprin’s work in 2002, but the message is the same; conditions specific to the laparoscopic surgical environment promote implantation and a more aggressive invasive capability for shed tumor cells. Thus, therapeutic use of laparoscopies in the resection of intra-abdominal malignancies deserves separate analysis, and although its introduction was fraught, it has lessons for us all.
Patient Factors and Positioning
Patient preparation has been modified over time. In the early days, nasogastric tube and urinary catheter were mandatory for diagnostic laparoscopy. In more recent times we have abandoned these in all but selected cases. We do request our patients to void their bladder once the attendant arrived to transport the patient to the operating room.
The operating theater setup depends on considering both patient and pathology factors. Our basic setup remains similar for the investigation of most emergency right iliac fossa (RIF) pains. The patient lies supine on a suitable gel-foam mattress and is secured by two table straps, one at the mid-thigh level and the other at the mid-chest. If significant pelvic pathology or a need for colonic resection is suspected, then the patient is placed on a bean bag and secured by a mid-chest strap (this allows more flexibility and access to the cervix if mobility is required).
Equipment Selection
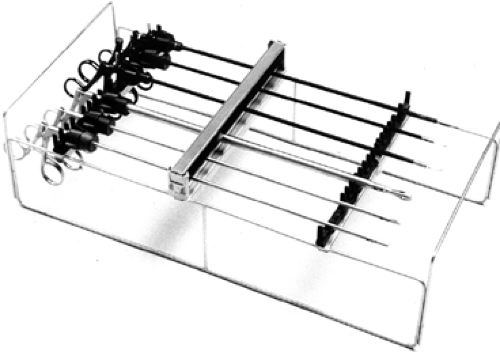
The basic laparoscopic set includes the laparoscope (either 5 or 10 mm), a range of graspers (including at least two atraumatic bowel graspers), and a scissors (Fig. 1). The use angled lens (30/45 degree) is desirable where possible, especially if pelvic or upper gastrointestinal (GI) conditions are strongly suspected preoperatively. This has the benefit of improved optical ability but relies upon the camera assistance having greater skill than with a straight scope. Ports should be selected depending on individual comfort and practice. Obesity is the main reason we amend our diagnostic instruments, favoring longer bariatric ports where the abdominal wall girth mandates. There is a similar setup for upper abdominal inflammatory processes, albeit with different positions of the monitors.
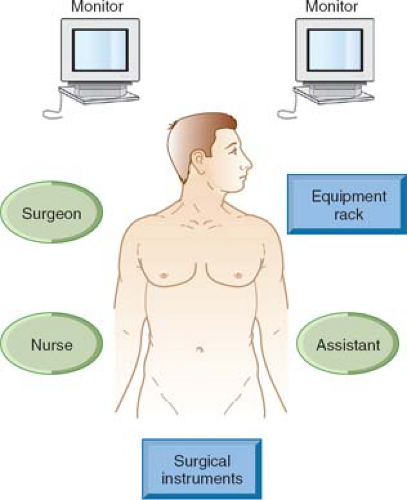
The laparoscopic stack (with contralateral slave monitor where possible) should be placed across from the right anterior superior iliac spine (ASIS). For upper abdominal conditions, the stack is best placed as per laparoscopic cholecystectomy at the right thorax. In addition, we routinely use the slave monitor on the contralateral side at the same level.
Frequently, the setup for these routine procedures is compromised in order to save time. The authors feel that this is a false economy as in the more difficult case this serves to severely increase the duration of the procedure. Standardization of setup is, in fact, the key to efficiency of setup. This is greatly facilitated if there is a “universal” setup for all different surgical teams within a department (Fig. 2).
Induction of the Pneumoperitoneum
Induction of the pneumoperitoneum is achieved by a modified open approach. Much has been written about the safety of the induction of the pneumoperitoneum by closed (Veress) or optical ports. In interpreting these data, it should be considered that the pneumoperitoneum induction method should be comfortable for the surgeon. It is our opinion that the open technique is the safest although our techniques differ slightly; neither of us
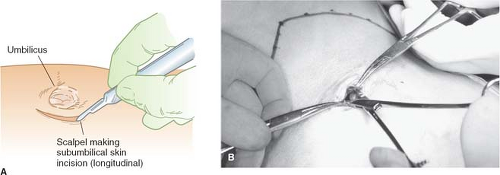
has experienced an injury caused by induction of the pneumoperitoneum in well over 4,000 laparoscopies. The technique involves dissection of the infraumbilical fascia at the base of the umbilical cicatrix using an artery forceps, placement of stay sutures (using polyglactin on a J needle) through the anterior fascia after vertical incision of the linea alba (Fig. 3). The posterior fascia is then incised under direct vision by electrocautery or bluntly using a finger depending on surgeon’s preference.
has experienced an injury caused by induction of the pneumoperitoneum in well over 4,000 laparoscopies. The technique involves dissection of the infraumbilical fascia at the base of the umbilical cicatrix using an artery forceps, placement of stay sutures (using polyglactin on a J needle) through the anterior fascia after vertical incision of the linea alba (Fig. 3). The posterior fascia is then incised under direct vision by electrocautery or bluntly using a finger depending on surgeon’s preference.
The needle closed technique was initially described by Veress in 1938. Although we do not routinely use it, it has had a resurgence of use in upper GI and bariatric surgery. With the patient in the Trendelenburg position, the Veress needle is inserted in the midline, below the umbilicus, aiming toward the pelvis at 45 degrees to the horizontal. Others prefer the left upper quadrant over the stomach. During insertion the abdominal wall should be grasped on either side, with towel clips, if necessary, and lifted away from the viscera. As the needle passes through the fascia and into the peritoneal cavity, the surgeon should feel a loss of resistance to the needle. The saline drop test indicates lack of resistance to flow and probable correct placement as the saline is sucked into the abdomen by the negative pressure occasioned by the lifted abdominal wall. The intra-abdominal pressures should be measured throughout; usually, they remain below 5 mm Hg. Initial insufflation should be set at a low flow rate until peritoneal entry is confirmed. Once adequate pneumoperitoneum is established a small skin incision is made in the midline below the umbilicus and a 10- to 12-mm trochar is then inserted in the same manner as the Veress needle. Trochars may have a spring-loaded “safety shield,” which protects the sharp edge of the trochar on entering the peritoneal cavity and locks in position to prevent organ injury.
Once the peritoneum is breached the port is secured and pressure is set to 12 to 15 mm Hg. We generally feel the lower end of the range adequate for purpose. The additional ports are placed according to pathology. We generally start with a suprapubic 5 mm with a 10 mm situated either in the midline (between the 5 mm and the optical) or in the left lower quadrant 2 cm medial and above the ASIS to standardize the sequence of the diagnostic part of the laparoscopy to aid completeness. In the case of RIF pain, the RIF is the first place to start with an anticlockwise direction to survey the other structures. The appendix is located following the teniae coli down to the base. It or its mesentery should not be grasped until the decision to resect has been reached. The ovaries, fallopian tubes, and uterus are then viewed, using a closed grasper to hook under the tubes to deliver the ovary. The left iliac fossa is then looked at to survey the colon. Then the small bowel is “run” from the cecum to the duodeno-jejunal flexure. This is to rule out segmental enteritis (inflammatory or Yersina enteritis) as well as a Meckel’s diverticulitis. The laparoscopy can be continued into the right upper quadrant to survey the duodenum, liver, and gallbladder, thus, completing the standard diagnostic sequence.
The placement of additional ports is governed by the operative plan made following the diagnostic part of the laparoscopy. Most disposable ports have excellent ridged profiles, which prevents the slippage associated with loosening cased by prolonged procedures. This has caused a reduction in the use of oversheaths in all but non-disposable ports.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree