Fig. 4.1.
Lymphoma with convoluted nuclei.
Nuclear inclusions exist in various forms
Viruses may be visualized by negative staining of whole particles or in thin sections. See section on nonneoplastic conditions for full discussion
It is important to differentiate virions from various other nuclear particulate inclusions that may occur:
Intranuclear glycogen may be seen in liver cells in diabetes, glycogen storage disease, Wilson disease, and hepatocellular tumors
Other particulate structures seen in nuclei include nuclear bodies and perichromatin granules
Furthermore, nuclear pores simulate virions for the unwary
♦
Nucleolus :
Multiple pleomorphic enlarged nucleoli are frequently seen in various malignant tumors
Anastomosing ropelike nucleolus is typical of seminoma (dysgerminoma)
The Cytoplasm
♦
Mitochondria may vary in crystal architecture, size, and number or may contain inclusions:
Tubulovesicular cristae are typically seen in steroid-producing cells, such as adrenal cortical adenoma (Fig. 4.2), Leydig cell tumor, ovarian hilus cell tumor, luteoma of pregnancy, thecoma, sex cord–stromal tumor, and granulosa cell tumor
Fig. 4.2.
Aldosteronoma (aldosterone-secreting adrenal cortical adenoma) demonstrating tubulovesicular cristae in mitochondria.
Platelike cristae are found in hepatocytes and tumors of same (Fig. 4.3)
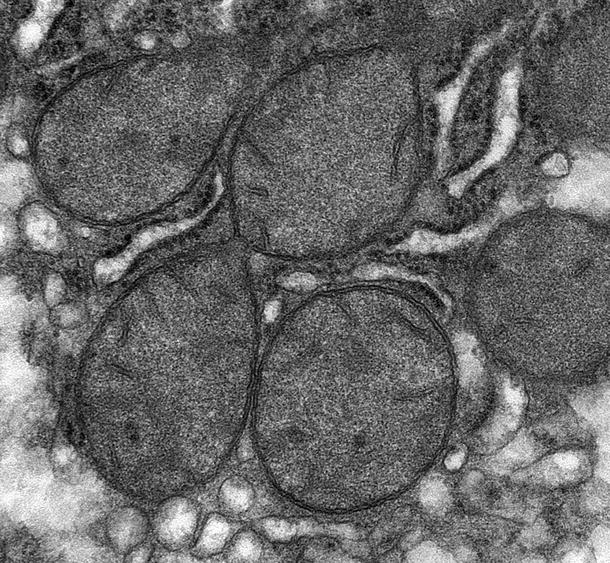
Fig. 4.3.
Hepatocellular adenoma with platelike cristae in mitochondria.
Giant mitochondria (megamitochondria) may be seen on occasion in any cell. They should only be considered significant when seen frequently in many cells:
Certain adenomas of the pituitary, thyroid, or salivary glands may contain megamitochondria
Secretory endometrial glands are an example of normal cells that may have giant mitochondria
Such mitochondria may also be associated with drug toxicity such as alcohol or cyclosporine
An increase in numbers of mitochondria is considered significant when the mitochondria push the other organelles to the side. Such cells are called oncocytes :
Oncocytoma of the thyroid gland (also called Hürthle cell neoplasm ), salivary gland, parathyroid, kidney (Fig. 4.4), and bronchial gland and Warthin tumor are examples
Fig. 4.4.
Oncocytoma of kidney with numerous mitochondria.
Mitochondria may contain various inclusions:
Small granules are frequently seen and represent divalent cations. An increase in the number of such granules may represent irreversible injury
Crystalline inclusions may be seen with drug injury (e.g., halothane, rosiglitazone), mitochondrial myopathies, etc
♦
Ribosomes and granular endoplasmic reticulum (GER) vary in amount depending on the state of the protein synthetic machinery:
Immature blastic or undifferentiated tumor cells show increased ribosomal rosettes. Examples include:
Ewing sarcoma, seminoma, and undifferentiated (lymphoepithelioma-like) carcinoma
Lymphoblastic lymphoma and Burkitt lymphoma
Increased GER is noted in cells synthesizing proteins for export:
Plasma cells, acinic cells, hepatocytes, fibroblasts, chondroblasts, and osteoblasts
And their neoplastic counterparts multiple myeloma, acinar cell carcinoma (salivary gland and pancreas), hepatocellular adenoma (Fig. 4.3) and carcinoma, fibroma, fibrosarcoma, chondroid tumors, and osteoblastic tumors
Tubuloreticular inclusions represent modified endoplasmic reticulum (Fig. 4.5):
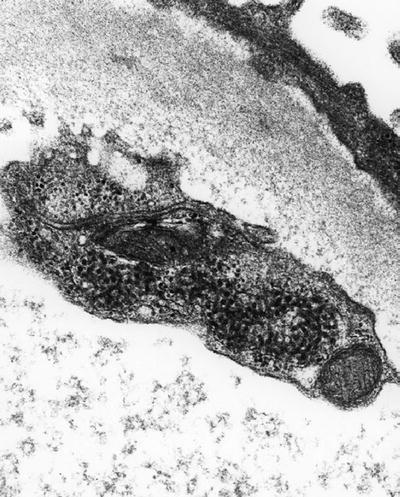
Fig. 4.5.
Tubuloreticular inclusion in endothelial cell from glomerular capillary loop.
They are seen in vascular endothelial cells, lymphocytes, and monocytes of patients with increased alpha and beta interferon
Examples include human immunodeficiency virus or other viruses, systemic lupus erythematosus, and scleroderma
♦
Increased smooth endoplasmic reticulum appears in steroid-secreting cells or as evidence of increased drug metabolism:
Steroid-secreting tumors include sex cord–stromal tumors, granulosa cell tumor, thecoma, Sertoli–Leydig cell tumor, hilus cell tumor of the ovary, Leydig cell tumor of the testis, and adrenal cortical tumors
Hepatocytes associated with protracted use of barbiturate also show increased smooth endoplasmic reticulum
♦
Glycogen is particulate and resembles ribosomes, although glycogen is somewhat larger. It is often washed away in routine preparation but leaves behind a characteristic appearance. It comes in two forms:
Beta glycogen; irregularly shaped 15–30 nm particles
Alpha glycogen; rosette-like aggregates (prominent in the liver)
Glycogen is abundant in Ewing sarcoma (Fig. 4.6); seminoma; rhabdomyosarcoma; yolk sac tumor; clear cell adenocarcinoma of the breast, vagina, endometrium, ovary (Fig. 4.7), salivary gland, and kidney; clear cell sarcoma; sugar tumor of the lung; and hepatocellular adenoma and carcinoma
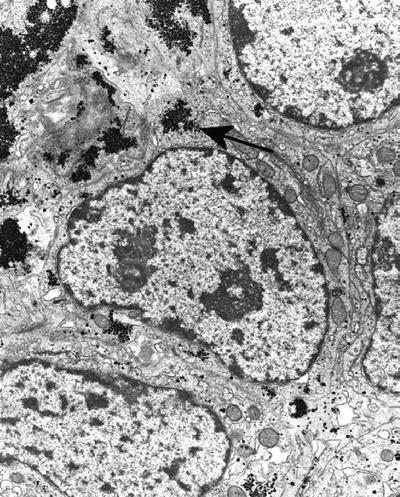
Fig. 4.6.
Ewing sarcoma showing glycogen (arrow).
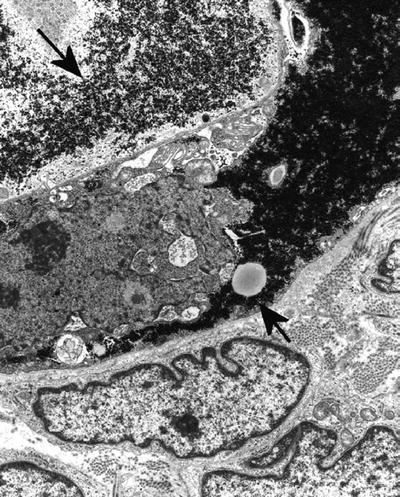
Fig. 4.7.
Clear cell adenocarcinoma of ovary showing glycogen (arrows) filling cells.
♦
Lysosomes have varied appearances depending on whether they are primary or secondary and on what material is present within their membrane:
Primary lysosomes measure 0.25–0.5 mm and are membrane-limited dense bodies that may be mistaken for neurosecretory dense-core granules. Lysosomes tend to be more heterogeneous in size and shape than neurosecretory granules
Secondary lysosomes are also known as autophagosomes, heterophagic vacuoles, or residual bodies and appear as lipofuscin pigment on light microscopy
Secondary lysosomes may contain intracellular metabolic products or phagocytosed extracellular material
Abundant lysosomes are seen in a variety of neoplasms or in lysosomal storage diseases:
Granular cell tumor (Fig. 4.8), granular cell ameloblastoma, thyroid follicular neoplasm, prostatic adenocarcinoma, and myelocytic or monocytic leukemia
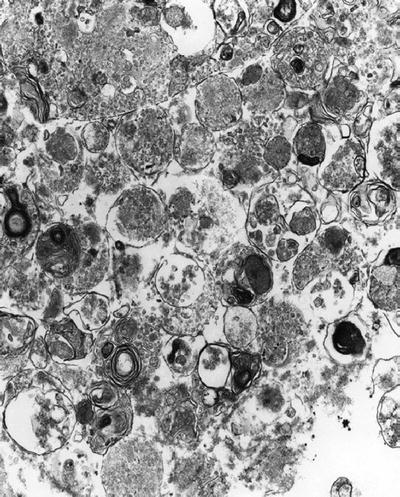
Fig. 4.8.
Granular cell tumor showing heterogeneity of lysosomal forms.
Genetically transmitted as autosomal recessive disease, for example, Tay–Sachs disease and Fabry disease (Fig. 4.9). See below for other examples of lysosomal storage disease
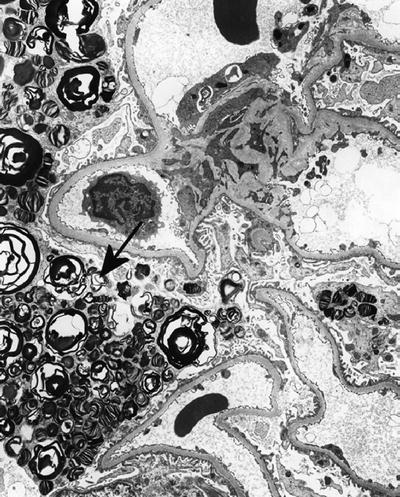
Fig. 4.9.
Fabry disease affecting glomerular podocyte with classic myelin-like appearance of storage material in lysosomes (arrow).
Michaelis–Gutmann bodies represent a specialized form of secondary lysosome seen in macrophages in malakoplakia. They are multilaminated, calcified spherules formed in secondary lysosomes, often containing calcium apatite crystals thought to represent bacterial remnants
♦
Neurosecretory, dense-core (or neuroendocrine) granules are membrane-limited dense-core granules that vary in size (50–400 nm), shape, and density depending on the protein contained in the granule but are uniform in appearance within a particular cell type. They usually have a halo between the core and the membrane:
Neuroblastoma (Fig. 4.10), esthesioneuroblastoma, and ganglioneuroblastoma contain 100 nm round dense-core granules that are more concentrated in cell processes than in cell bodies
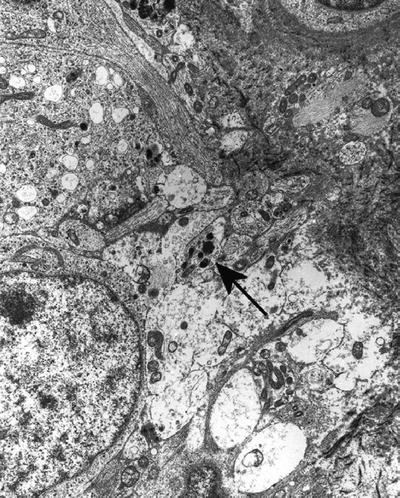
Fig. 4.10.
Neuroblastoma showing more neurosecretory granules (arrow) in processes than in cell bodies.
Pheochromocytoma (Fig. 4.11) has two cell types, one with eccentric dense cores (norepinephrine) and the other (epinephrine) with centrally located spherical cores, resembling the granules of neuroblastoma
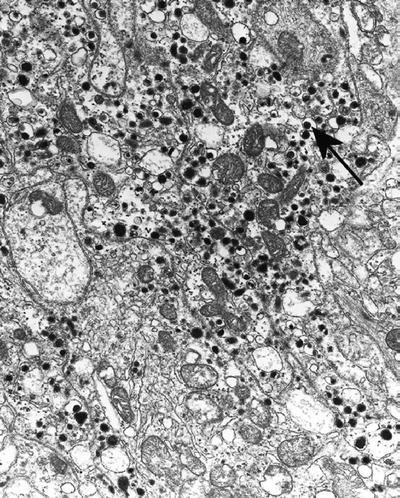
Fig. 4.11.
Pheochromocytoma containing norepinephrine granules with eccentric cores (arrow) and epinephrine granules (all others).
Paraganglioma has spherical granules that measure 100–200 nm
Carcinoid tumor (Fig. 4.12) contains serotonin-secreting pleomorphic granules
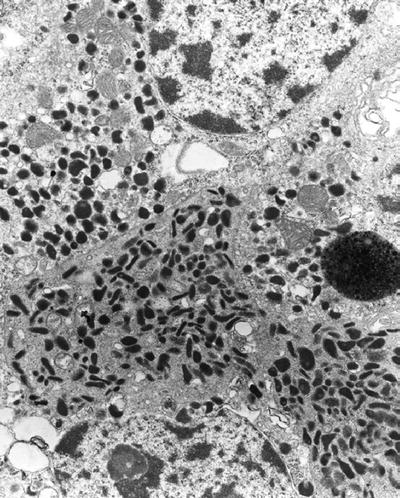
Fig. 4.12.
Carcinoid tumor with pleomorphic granules.
Medullary thyroid carcinoma has two types of calcitonin-containing granules. Type 1 is large and medium dense with no halo, and type 2 is small and very dense and has a halo
Merkel cell tumor (cutaneous neuroendocrine carcinoma) contains small spherical granules (115–200 nm)
Small- (Fig. 4.13) and large-cell neuroendocrine carcinoma contains sparse dense-core granules
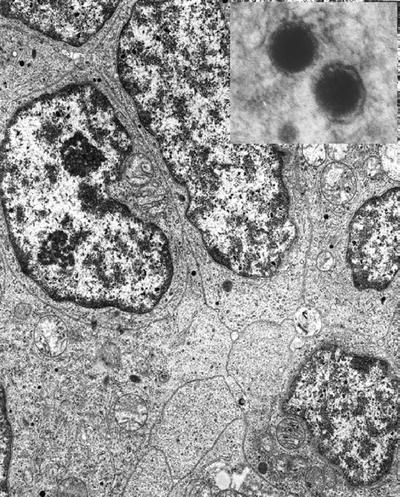
Fig. 4.13.
Small-cell neuroendocrine carcinoma with sparse neurosecretory granules . Inset shows dense core with halo.
Renal juxtaglomerular cell tumor demonstrates spherical or rhomboid granules
♦
Secretory granules of normal and neoplastic exocrine glands are of two types:
Mucous granules (0.7 − 1.8 μm) are membrane-limited, granular, reticulated, or flocculent material of various densities (Fig. 4.14)
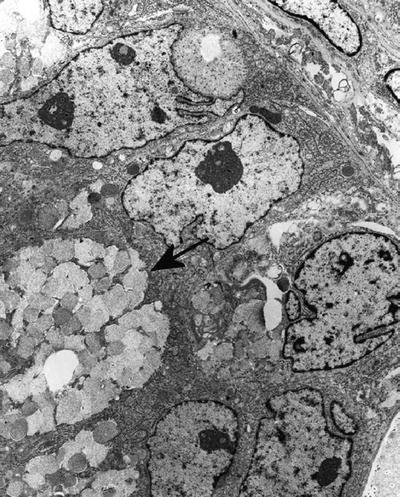
Fig. 4.14.
Adenocarcinoma of lungs, mucin cell type , showing mucous granules (arrow).
Electron microscopy does not differentiate various types of mucins; for this purpose, histochemical and immunohistochemical studies are needed
Serous or zymogen granules (0.5–1.5 μm) are membrane-limited, dense granular matrix that may or may not contain enzymes
Serous or zymogen granules are seen in pancreatic acinar cells, serous acinic cells of the salivary gland (Fig. 4.15) or of the upper respiratory tract, gastric chief cells, and Paneth cells
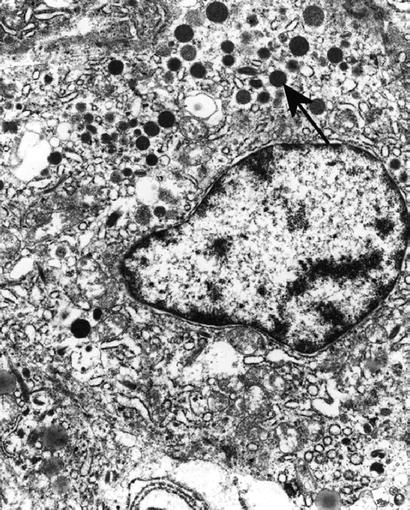
Fig. 4.15.
Acinic cell carcinoma with zymogen granules (arrow).
♦
Lipid droplets are not limited by a membrane and may be seen in virtually any kind of cell in small numbers. More abundant lipid droplets are seen in the following conditions:
Adipose tissue, normal and neoplastic, contains large droplets
Hibernomas contain abundant lipid droplets and numerous mitochondria
Steroid-secreting tumors – thecoma, ovarian hilus cell tumor, Leydig cell tumor, and adrenal cortical tumors – contain numerous lipid droplets
Numerous lipid droplets are also frequently observed in sebaceous gland tumor, renal clear cell carcinoma, xanthoma, and fibrohistiocytoma
♦
Microtubules and numerous types of cytoplasmic fibrils/filaments may be observed:
Microtubules are 25 nm in diameter and are seen in a variety of cells:
Axonemes of cilia are composed of 9 + 2 doublets in motile cilia (Fig. 4.16) and 9 + 0 in stereocilia
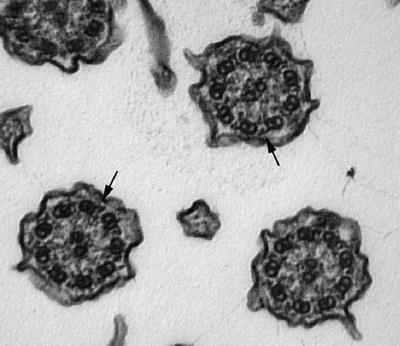
Fig. 4.16.
Cross sections of respiratory cilia showing the 9 + 2 microtubular pattern . Note also dynein arms (arrows).
Neurotubules are noted in neuronal cell tumors (neuroblastoma, ganglioneuroma, primitive neuroectodermal tumor) particularly in their processes
Intracisternal microtubules have been described in melanoma and myxoid chondrosarcoma
Microtubules may also be seen in platelets in a circumferential band
Scattered microtubules have been described in many cell types
Filaments or microfibrils are seen in different sizes:
Thick filaments are usually skeletal muscle myosin and measure 15 nm wide
Intermediate filaments measure 10–12 nm wide and are composed of many different proteins including cytokeratin, vimentin, desmin, glial filaments, and neurofilaments:
◦
With the exception of tonofibrils (cytokeratin forming characteristic dense curvilinear bundles (Fig. 4.17)), they are not distinguishable by electron microscopy
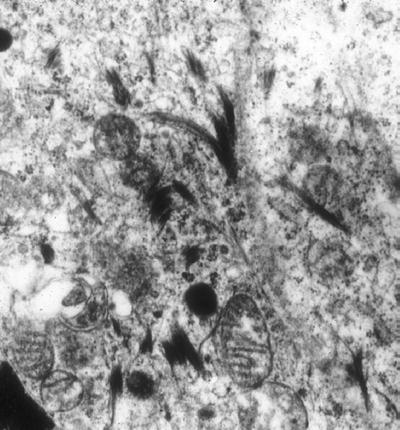
Fig. 4.17.
Squamous cell carcinoma showing typical curved tonofilaments.
Actin microfilaments measure 6–7 nm wide and are found in smooth and striated muscle cells, myofibroblasts, and myoepithelial cells
Filamentous inclusions may be seen in certain conditions:
Nemaline rod is a threadlike or oblong structure having a latticelike appearance that may be found beneath the sarcolemma of skeletal muscle:
They were first described in nemaline myopathy but have also been seen in muscular dystrophy and polymyositis
Mallory hyaline or body is an irregularly shaped accumulation of cytokeratin intermediate filaments in liver cells:
They may be seen in alcoholic and nonalcoholic steatohepatitis, Wilson disease, and various cholestatic conditions
They have also been described in hepatocellular carcinoma
♦
Certain organelles are specific to particular cells or a group of cells:
Crystals of Reinke (Fig. 4.18) are hexagonal prism-like, highly ordered 10 nm thick filaments that are not limited by a membrane and are seen in Leydig cells and ovarian hilus cells. These crystals are found in 35% of Leydig cell tumors
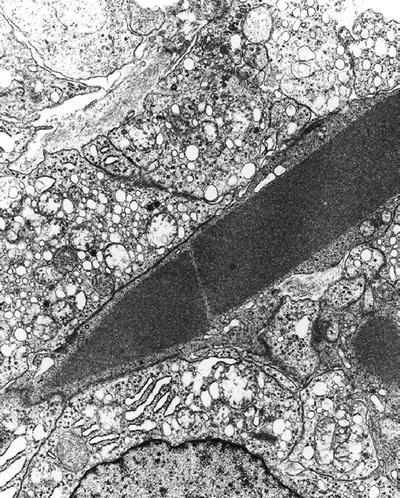
Fig. 4.18.
Leydig cell tumor showing Reinke crystal .
Charcot–Böttcher “crystalloids” are closely packed, electron-dense, longitudinal arrays of fibrils or tubules lacking a geometric crystalline lattice in postpuberty Sertoli cells. They are described in an occasional Sertoli cell tumor
Langerhans cell granule (Birbeck granule) (Fig. 4.19) is a 34 nm wide rod-shaped structure of variable length with a periodic or striated zipper-like core. The limiting membrane is often dilated at one end giving the granule a tennis racketlike appearance:
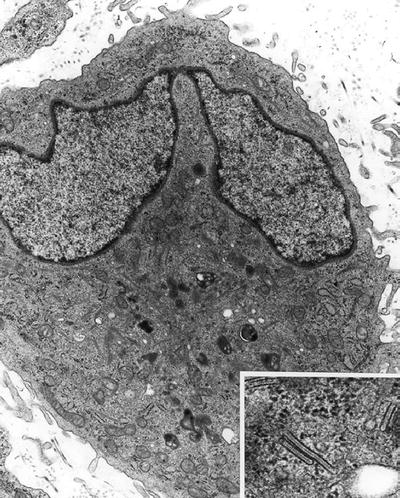
Fig. 4.19.
Langerhans histiocyte . Inset shows Birbeck granules.
These structures are seen in Langerhans cell histiocytosis (eosinophilic granuloma, Letterer–Siwe disease, or Hand–Schüller–Christian disease)
They are absent in follicular dendritic and interdigitating dendritic cells
Melanosomes (Fig. 4.20) are seen in cells that produce melanin:
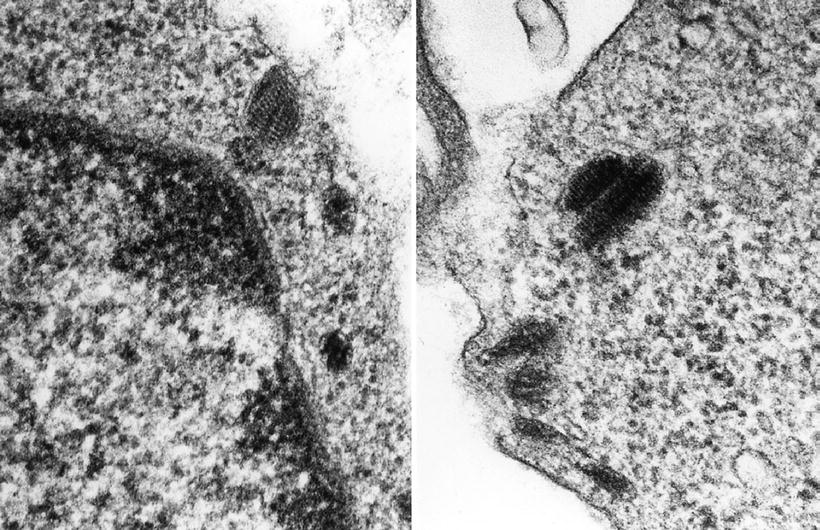
Fig. 4.20.
Melanosomes are shown. The left panel shows a premelanosome prior to pigment accrual. The right panel shows a melanized melanosome.
They mature stepwise in four stages with stage II/III premelanosomes as most characteristic, consisting of ovoid or ellipsoidal vesicles with striated internal structures
They are seen in melanocytic neoplasms, but stage II and III premelanosomes may also be seen in unpigmented variants of melanocytic neoplasms and may be aberrant
Weibel–Palade body (Fig. 4.21) is a rod-shaped body with microtubular internal structures embedded in dense matrix seen in blood vessel endothelial cells but not in lymphatic endothelial cells:
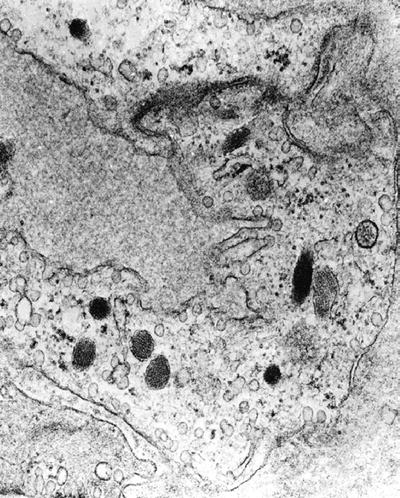
Fig. 4.21.
Weibel–Palade bodies may be seen in endothelial cells.
They are easily found in benign vasoformative tumors and epithelioid hemangioendothelioma
They are only rarely found in malignant vasoformative tumors, such as angiosarcoma or Kaposi sarcoma, reflecting the lack of differentiation
♦
Certain other organelles are uncommon and are not typical of any particular cell type although they are seen in particular disorders:
Ribosome–lamella complex consists of several layers of parallel cylindrical lamellae, separated by ribosome-like granules:
It is most commonly seen in patients with hairy cell leukemia
It is also described in various other neoplasms and disorders, for example, chronic lymphocytic leukemia, various lymphomas, reactive lymph node, adrenal cortical adenoma, insulinoma, Sertoli cell tumor, meningioma, and glioma
Lamellar inclusion body (myelinosome) (Fig. 4.22) is a concentrically arranged, stacked electron-dense membrane and represents the surfactant in type II alveolar cells:
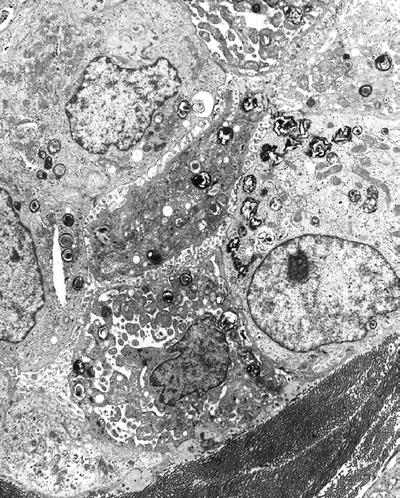
Fig. 4.22.
Lamellar inclusion bodies are noted in a type II alveolar lining cell and represent surfactant.
This change is also seen as a metabolic product of many drugs including amiodarone and gentamicin
Similar structures are also seen in various lysosomal storage diseases
Spironolactone bodies resemble surfactant myelinosomes and are seen in zona granulosa cells of patients treated with the aldosterone antagonist, spironolactone
The Cell Surface (Plasmalemma)
♦
Interdigitating complex cell membranes are common in glandular epithelial tumors (gastrointestinal adenocarcinomas, parathyroid adenomas, and sweat gland tumors), epithelioid sarcoma, meningothelial meningioma, and schwannoma
♦
Cilia may be motile (9 + 2 microtubular doublets) or may not move (stereocilia with 9 + 0 microtubular doublets)
♦
They are present on the luminal surfaces of many types of epithelial cells
♦
Cilia are lost in malignant tumors. Various abnormalities of the cilia are described in benign epithelial tumors. They are not useful in differential diagnosis
♦
Pinocytotic vesicles are seen in well-differentiated smooth muscle, vascular endothelial, and perineurial tumors
♦
Microvilli are a surface specialization that increases the surface area of an epithelial cell bordering a lumen. They characteristically contain anchoring rootlets and are covered by a glycocalyx:
Closely packed rigid microvilli (brush border) are seen in adenocarcinoma of the gastrointestinal tract and intestinal-type adenocarcinoma of other sites
Long, branching, shaggy microvilli are typical of mesothelial cells and mesothelioma (Fig. 4.23) (epithelial type)
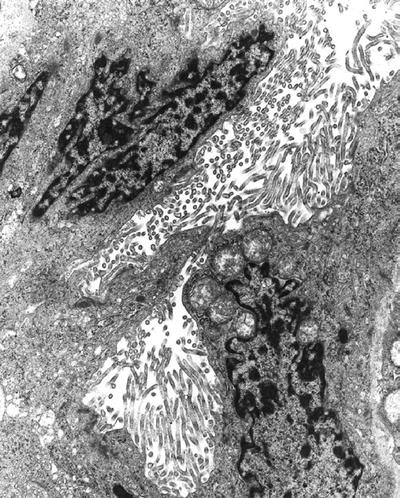
Fig. 4.23.
Mesothelioma is characterized by long bushy microvilli.
♦
Intracytoplasmic lumens are vacuolar-like structures lined by scattered microvilli :
Get Clinical Tree app for offline access

They are a useful marker for adenocarcinoma
They are particularly prominent in breast carcinoma (Fig. 4.24) (both ductal and lobular)
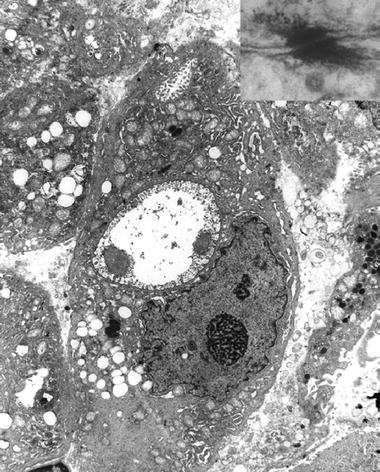
Fig. 4.24.
Ductal breast carcinoma shows two intracellular lumina with microvilli . (Inset) A desmosome seen at high magnification represents a “spot-weld” between two cells.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


