Characteristics
Partial mole
Early complete mole
Complete mole
Ploidy
3 N
2 N
2 N
Villous p57KIP2 positivity
+
−
−
Dimorphic villous populations
+
−
−
Dysmorphic villi
+
−
−
“Cauliflower-like” villous branching
−
+
−
Hypercellular villous stroma
−
+
−
Villous stromal karyorrhexis
−
+
−
Uniformly hydropic villi
−
−
+
Villous trophoblast hyperplasia
+
+
++
Implantation site trophoblast atypia
+
+
+
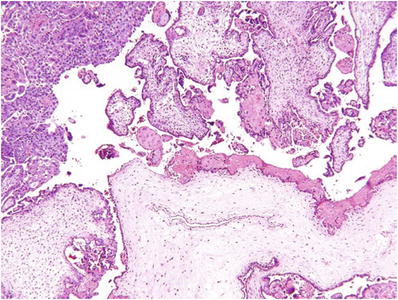
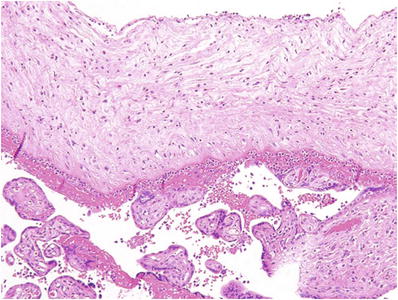
Fig. 33.1.
Partial hydatidiform mole.
Villous trophoblast hyperplasia (usually predominantly syncytiotrophoblast)
Dimorphic population of large and small villi without intermediate forms
Hydropic villi >0.5 mm
Irregularly irregular villous contour (“coast of Norway fjord-like infoldings”)
•
Often with multicellular trophoblast inclusions within a villous stroma
♦
Other helpful histologic features not observed in all cases include:
Maze-like villous capillary vascular pattern in the villous stroma
Atypical implantation site trophoblast (enlarged irregularly shaped hyperchromatic nuclei)
Molar villi with well-delineated central cisterns
Villous stromal karyomegaly (individual stromal cell nuclei 2–3 × normal size)
Ancillary Studies
♦
Flow cytometry, image analysis, or fluorescence in situ hybridization (FISH) can occasionally be helpful for differentiating partial versus complete mole or partial mole versus hydropic abortus (modal DNA index = 1.5 (PM) versus 1.0 and 2.0, respectively, for the other two conditions)
Differential Diagnosis
♦
Hydropic abortus (HA)
These gestations present clinically as anembryonic pregnancies or missed abortions
They usually lack embryonic and other placental adnexal structures (yolk sac, umbilical cord, amnion) and often have an abnormal karyotype
They lack one or more of the criteria required to diagnose PM
Focal trophoblast hyperplasia can be seen in approximately 3% of nonmolar abortions, which can be followed up by a single maternal hCG titer to ensure return to baseline
♦
Complete hydatidiform moles (CMs)
CMs have uniformly hydropic villi with central cisterns
They lack the second population of small villi seen in PM
Villous trophoblast hyperplasia is generally more diffuse and involves cytotrophoblast and syncytiotrophoblast equally
p57KIP2 immunostaining can be useful in distinguishing PM from CM (see below)
Complete Hydatidiform Moles
Clinical
♦
CMs are diandric diploid gestations (two sets of chromosomes with both sets derived from the father) derived from fertilization of a maternal ovum lacking the female pronucleus by either two sperm or, more commonly, a single sperm followed by endoduplication
♦
As in PM, trophoblast hyperplasia in CM results from an overexpression of paternally derived gene products and/or a lack of maternally derived gene products (genomic imprinting)
♦
CMs present clinically in two distinct scenarios:
Classic CM (>8 weeks), various combinations of the following clinical signs and symptoms:
•
Uterus large for dates, vaginal bleeding, elevated maternal hCG titers, hyperemesis, preeclampsia, thyrotoxicosis, bilateral adnexal masses (theca lutein cysts)
Early CM (<8 weeks), anembryonic missed abortion by ultrasound
♦
Clinical management:
Serial maternal serum hCG titers are measured for 1 year following diagnosis
Metastatic workup is initiated and chemotherapy begun if levels plateau or rise
CM requires chemotherapy in 20–30% of cases
The risk of subsequent choriocarcinoma is much higher than for PM, approximately 1/40 without treatment
Gross Findings
♦
Classic CM usually consists of a discohesive collection of swollen grape-like molar villi up to 5–10 mm in diameter
♦
Early CM is grossly unremarkable
Microscopic
♦
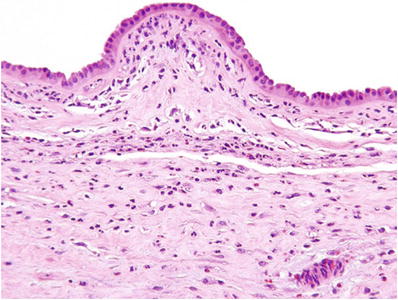
Classic (>8 weeks) CM (Fig. 33.2)
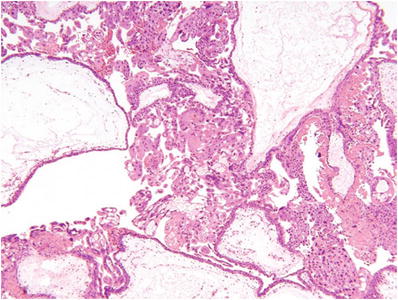

Fig. 33.2.
Complete hydatidiform mole.
Diffuse and circumferential villous trophoblast hyperplasia
Involves both cytotrophoblast and syncytiotrophoblast
Uniformly hydropic villi and many with well-defined central cisterns
Lack of fetal vessels and nucleated red blood cells
Frequent atypical implantation site
♦
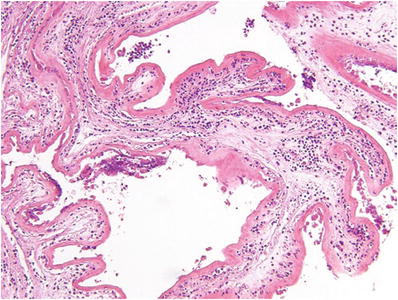
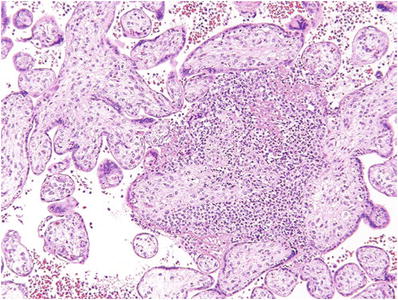
Early (<8 weeks) CM (Fig. 33.3)
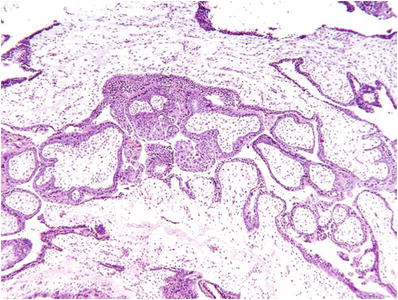

Fig. 33.3.
Early complete hydatidiform mole.
Focal cytotrophoblast and syncytiotrophoblast hyperplasia of both villi and the undersurface of the chorion
Redundant bulbous terminal villi (+focal hydrops)
Hypercellular myxoid villous stroma often with stromal karyorrhexis
Labyrinthine network of villous stromal canaliculi
Atypical implantation site
Fetal vessels or nucleated red blood cells may rarely be seen
Immunocytochemistry
♦
p57KIP2 immunostaining is negative in the nuclei of villous stromal and cytotrophoblast cells in CM
p57KIP2 is a paternally imprinted gene expressed only by the female genome which is lacking in diandric gestations
Of note, the gene is not imprinted and hence is expressed in extravillous trophoblast from CM
DNA Studies
♦
PCR-based microsatellite analysis on microdissected maternal and fetal tissue removed from the paraffin blocks can be used to confirm diandric origin
Differential Diagnosis
♦
PM: The differential diagnosis with CM is discussed above
p57KIP2 staining is positive due to the presence of the female genome
♦
Mesenchymal dysplasia: Enlarged villi with stromal-vascular overgrowth and central fluid-filled cisterns. These cases have diandric stroma but biparental trophoblast
Unlike classic CM, these gestations generally occur in the second half of pregnancy and lack trophoblast hyperplasia
p57KIP2 staining is negative in stromal cells but positive in trophoblast
Rare cases will show mosaicism leading to areas of mesenchymal dysplasia, classic HM, and normal villi coexisting in the same placenta
♦
Hydropic abortions (HA) generally lack any villous trophoblast hyperplasia or atypical implantation site trophoblast
Unlike early CM, they show degenerative features such as hypocellular villous stroma and intervillous fibrin
They are p57KIP2 positive
♦
Nonhydropic abortions (threatened, incomplete, complete, and elective) also lack villous trophoblast hyperplasia and atypical implantation site
While they lack the degenerative features seen with HA, they do not show the stromal hypercellularity and canaliculi seen with early CM or the cisterns associated with classic HM
They are also p57KIP2 positive
Malignant Trophoblastic Tumors
Choriocarcinoma
Clinical
♦
Choriocarcinoma (CC) is a rapidly growing, highly invasive malignancy with frequent metastases
♦
Although historically associated with a high mortality rate, CC is now generally curable with multiagent chemotherapy
♦
CC usually presents as a uterine tumor or in a metastatic site
♦
It can develop in any pregnancy at any stage
♦
The relative frequency of different categories of preceding pregnancies is as follows:
Hydatidiform mole (45%)
Term pregnancy (25%)
Spontaneous abortion (25%)
Ectopic pregnancy (5%)
♦
Intraplacental choriocarcinoma is a special category of early CC detected grossly as a hemorrhagic nodule or microscopically as a hemorrhagic/necrotic lesion with sparse diagnostic foci of CC at the periphery
♦
Despite the early stage, these lesions may be associated with both maternal and fetal metastases
♦
Clinical Staging
♦
Elevated hCG only
♦
Uterine corpus involvement
♦
Lung metastases
♦
Pelvic and/or vaginal metastasis
♦
Distant metastasis
♦
Adverse prognostic factors include:
Older age
Preceding nonmolar pregnancy
Longer interval to preceding pregnancy
Tumor size >5 cm
hCG level >105 mIU/L
Number of metastases
Metastasis to brain, liver, or GI tract
Previous chemotherapy
Gross Findings
♦
CC is a grossly hemorrhagic, ill-defined lesion within the uterovaginal wall or in the parenchyma of other organs
♦
Hemorrhage and necrosis are sometimes so extensive that it is difficult to detect the viable tumor
Microscopic
♦
CC has a biphasic pattern consisting of malignant villous cytotrophoblast and syncytiotrophoblast
♦
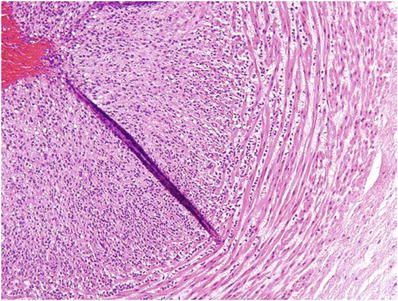
The classic architectural pattern (Fig. 33.4) consists of groups of 10–50 cytotrophoblasts with scant clear cytoplasm and uniformly enlarged central nuclei with prominent nucleoli and prominent margination of chromatin
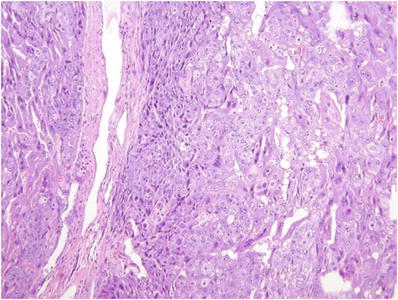

Fig. 33.4.
Choriocarcin oma.
♦
These clusters are surrounded by a wreath-like arcade of multinucleate syncytiotrophoblast with enlarged irregular hyperchromatic nuclei and purple cytoplasm
♦
The presence of extensive tumor-associated hemorrhage, necrosis, and perpendicular invasion of myometrial fibers is sometimes helpful in the absence of classic pattern described above
Immunocytochemistry
♦
Malignant cytotrophoblast is positive for cytokeratin only
♦
Malignant syncytiotrophoblast expresses cytokeratin, hCG, human placental lactogen (hPL), and placental alkaline phosphatase (PLAP)
♦
High Ki-67 proliferation index (>90%)
Differential Diagnosis
♦
Intervillous X-cell nodules
These lesions need to be distinguished from intraplacental CC
They are aggregates of extravillous trophoblasts
They lack nuclear atypia and necrosis, have dense eosinophilic cytoplasm, are surrounded by a prominent fibrinoid matrix, and express intermediate or epithelioid trophoblast markers such as inhibin A, p63, and Mel-CAM
♦
Placental site trophoblastic tumor (PSTT)
These tumors are less hemorrhagic than CC at gross examination
Tumor cells have a dense eosinophilic cytoplasm and larger, more irregular, and hyperchromatic nuclei
Multinucleate cells are rare
Tumor cells stain with intermediate trophoblast markers including hPL, Mel-CAM, HLA-G, and cytokeratin
Intermediate Ki-67 proliferation index (10–30%)
♦
Epithelioid trophoblastic tumors (ETTs)
These tumors also lack extensive hemorrhage and necrosis
They are composed of predominantly mononuclear cells with a clear, mildly eosinophilic cytoplasm, extracellular keratin-like pearls, and immunocytochemical features of epithelioid trophoblast such as HLA-G, inhibin A, and p63
Variable Ki-67 proliferation index (>10%)
Placental Site Trophoblastic Tumors
Clinical
♦
Placental site trophoblastic tumors (PSTT ) are rare, generally indolent tumors composed of intermediate trophoblast usually presenting within the uterus
♦
They usually follow term pregnancies (95% of cases), often with a long interval (up to 15 years)
♦
Local invasion and distant metastasis are observed in 15–20% of cases, and these invasive tumors are often resistant to chemotherapy
♦
Unlike CC, PSTT generally present with a low serum hCG level, generally <2,500 mIU/mL
♦
The precursor lesion for PSTT is unknown
PSTT rarely follow molar pregnancy
Placental site nodules, commonly seen in the curettings of multiparous patients, are potential precursors
♦
Virtually, all PSTT have a 46, XX karyotype, suggesting that they may have arisen from mosaic diandric intermediate trophoblast or as consequence of defective X inactivation in biparental intermediate trophoblast
♦
Since trophoblast generally manifests preferential inactivation of the paternal X chromosome, both could be associated with anomalous expression of paternal X-linked genes
Gross Findings
♦
PSTT usually present as nodular or polypoid masses
♦
Occasionally, it may be diffusely infiltrative
♦
Extensive hemorrhage or necrosis is uncommon
Microscopic
♦
PSTT are composed of cohesive sheets of mononuclear intermediate trophoblasts (Fig. 33.5)
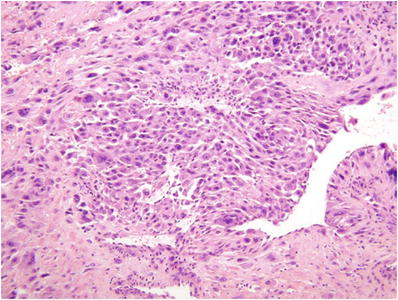

Fig. 33.5.
Placental site trophoblastic tumor (PSTT).
Aggregates of neoplastic cells may not only be associated with zonal necrosis but also show prominent arterial remodeling recapitulating the appearance of the normal implantation site
Individual tumor cells are generally mononuclear, but occasionally binucleate, and have abundant eosinophilic cytoplasm
Nuclei are enlarged with coarse clumped chromatin and prominent nucleoli
♦
Tumors with a mitotic rate >5/10 high-power fields, prominent necrosis, and less dense eosinophilic cytoplasm have a worse prognosis
Immunocytochemistry
♦
Tumor cells have an intermediate trophoblast phenotype: keratin, hPL, HLA-G, and Mel-CAM positive; hCG and inhibin A, weak or focally positive; p63 negative
♦
Ki-67 proliferation index generally 10–30%
Differential Diagnosis
♦
Placental site nodule (PSN) and plaque
These “lesions” represent involuting/remote implantation sites
•
They are well-circumscribed aggregates of intermediate trophoblast embedded in a fibrinoid matrix and may be seen following any term pregnancy
•
Unlike PSTT, they have low cellularity and bland cytologic features
•
Immunocytochemical staining shows an epithelioid trophoblast phenotype (HLA-G, p63 positive; hPL, hCG negative)
•
Low Ki-67 proliferation index (<8%)
♦
Exaggerated placental site
This “lesion” is simply a normal cellular implantation site that may be encountered in any abortion specimen (spontaneous or elective)
•
In this process, the intermediate trophoblast infiltrates the decidua and myometrium as single or small groups of cells
•
Unlike PSTT, multinucleate placental site giant cells are present in the specimen
•
There is no evidence of necrosis or destructive myometrial invasion
•
This pattern is not seen following term pregnancies
Epithelioid Trophoblastic Tumor
Clinical
♦
ETT is a rare tumor of the uterus or cervix, generally presenting with vaginal bleeding
Extrauterine disease is not uncommon
Preceding GTD is noted in 50% of cases
♦
Tumors resembling ETT can also develop from resistant nodules within a previously treated CC
♦
ETT is associated with an hCG level intermediate between that seen with CC and PSTT, generally <10,000 mIU/mL
Gross Findings
♦
ETT presents as a uterine or cervical mass, usually nodular without extensive hemorrhage or necrosis
Microscopic
♦
ETTs are composed of cords and nests of mononuclear extravillous trophoblast with clear or eosinophilic cytoplasm (Fig. 33.6)
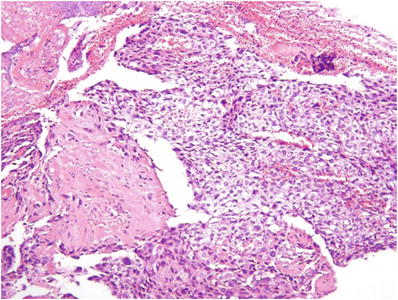

Fig. 33.6.
Epithelioid trophoblastic tumor (ETT).
The overall appearance is reminiscent of the trophoblast in the chorion laeve of the placental membranes (so-called epithelioid trophoblast)
♦
Tumor cell aggregates are often associated with eosinophilic, fibrillar, hyaline-like material resembling keratin
♦
ETT may occasionally grow along the surface of endocervical glands
Immunocytochemistry
♦
Tumor cells have an epithelioid trophoblast phenotype (cytokeratin, p63, inhibin A, HLA-G positive; Mel-CAM, hPL, and hCG weak to negative)
♦
Ki-67 proliferation index >10%
Differential Diagnosis
♦
CC
ETTs are generally nonhemorrhagic and are associated with a lower hCG titer
Tumors lack multinucleate syncytiotrophoblast
Mononuclear tumor cells have more abundant cytoplasm than cytotrophoblast in CC
♦
PSTT
ETTs more commonly involve the cervix and lower uterine segment
hCG titers are usually higher
Individual tumor cells are smaller, lack strongly eosinophilic cytoplasm, and have a higher N/C ratio than intermediate trophoblast in PSTT
Implantation site-like arterial remodeling is absent
Immunostains for hPL, inhibin A, and Mel-CAM are negative or focally positive
♦
Squamous cell carcinoma
ETT lacks positivity for human papillomavirus (HPV) and expresses cytokeratin 18 and p63
The overlying epithelium lacks squamous atypia
♦
Epithelioid leiomyosarcoma
ETT lacks positivity for smooth muscle markers and expresses low and high molecular weight keratin filaments
More spindled typical smooth muscle cells are absent
Placenta
Acute Chorioamnionitis
Clinical
♦
Acute chorioamnionitis (ACA), also known as the amniotic fluid infection syndrome, is the leading cause of preterm birth
♦
Up to 60% of infants <1.5 kg have histologic ACA in their placentas
♦
ACA is also a risk factor for cerebral palsy, particularly when associated with an intense fetal inflammatory response as assessed histologically or by markedly increased cytokines in fetal circulation
♦
ACA is usually an ascending infection caused by organisms resident in the cervicovaginal tract
Less commonly, organisms may spread to the placental membranes via hematogenous seeding (e.g., periodontal infections) or by transuterine contiguous spread from other pelvic organs (e.g., the bladder, fallopian tube)
♦
Risk factors for ascending infection include cervical dilatation secondary to either incompetent cervix or preterm labor and changes in cervicovaginal flora as in bacterial vaginosis, foreign bodies (cerclage, IUD), or colonization by group B Streptococci
♦
Causative organisms include the normal flora of the cervicovaginal tract, particularly anaerobic bacteria and Mycoplasma spp
Less commonly, group B Streptococci, E. coli, and other aerobic bacteria are implicated
The presence of these latter organisms increases the risk of spread to the fetus (early onset sepsis)
With foreign bodies, Candida spp. become more frequent
Rare, highly virulent causes of ACA include Listeria monocytogenes and Campylobacter fetus
Gross Findings
♦
The fetal surface of the placenta may show grayish discoloration with haziness and blurring of the underlying chorionic plate vessels
Severe ACA can be associated with diffuse yellow-green exudate
♦
Candida ACA is characterized by yellow/white microabscesses on the surface of the umbilical cord
♦
L. monocytogenes and C. fetus may be associated with irregular pale yellow intervillous abscesses or septic infarcts on the cut surface of the villous parenchyma
Microscopic
♦
The inflammatory response to infection in ACA is diffuse
A diagnosis of focal ACA is not recognized
The infiltrate is composed of polymorphonuclear leukocytes (PMNs) with occasional eosinophils or metamyelocytes, particularly in premature gestations
Prolonged low-level infection may also elicit a subpopulation of vacuolated macrophages in the chorionic plate (subacute [chronic] chorioamnionitis)
♦
Maternal inflammatory response
Membranes (PMN from decidual venules)/chorionic plate (PMN from intervillous space)
♦
Fetal inflammatory response
Chorionic plate (PMN from chorionic vessels)/umbilical cord (PMN from umbilical vessels)
•
Early (Fig. 33.10): PMNs in the chorionic vessels and/or umbilical vein
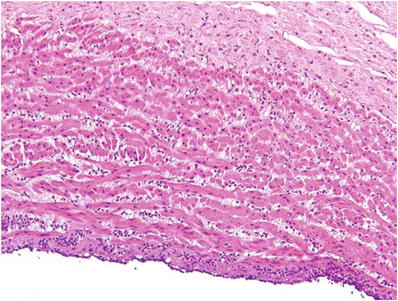
Fig. 33.10.
Acute umbilical phlebitis.
•
•
Late (Fig. 33.12): Degenerating PMNs in Wharton jelly surrounding umbilical vessels
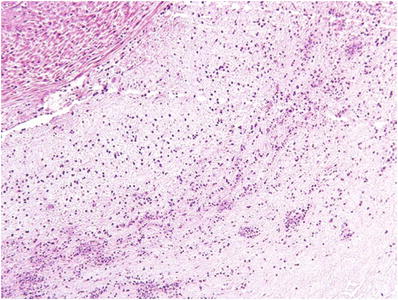
Fig. 33.12.
Necrotizing funisitis (acute concentric perivasculitis).
•
Severe fetal inflammatory response (Figs. 33.13 and 33.14): Near confluent PMN in fetal vessels wall, usually with signs of vessel wall damage or early mural thrombosis
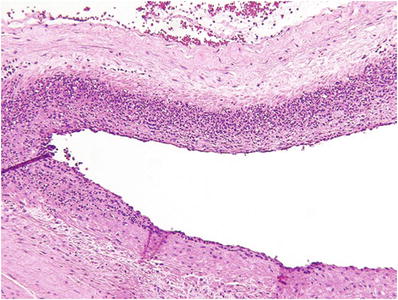
Fig. 33.13.
Severe fetal vasculitis (intense chorionic vasculitis).
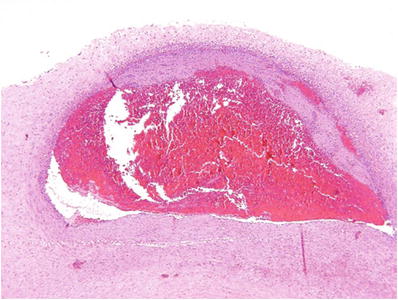
Fig. 33.14.
Fetal vasculitis with nonocclusive chorionic vessel thrombus.
♦
Candida spp
Peripheral funisitis with neutrophilic microabscesses (Fig. 33.15)
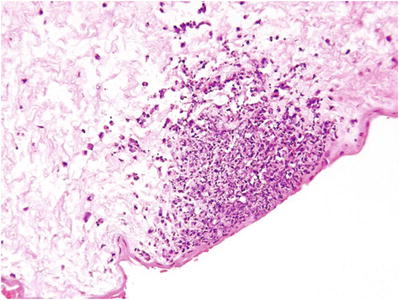
Fig. 33.15.
Peripheral funisitis (Candida spp.).
♦
Get Clinical Tree app for offline access

L. monocytogenes/C. fetus
Acute intervillositis with intervillous abscesses (Fig. 33.16)