Patient Story
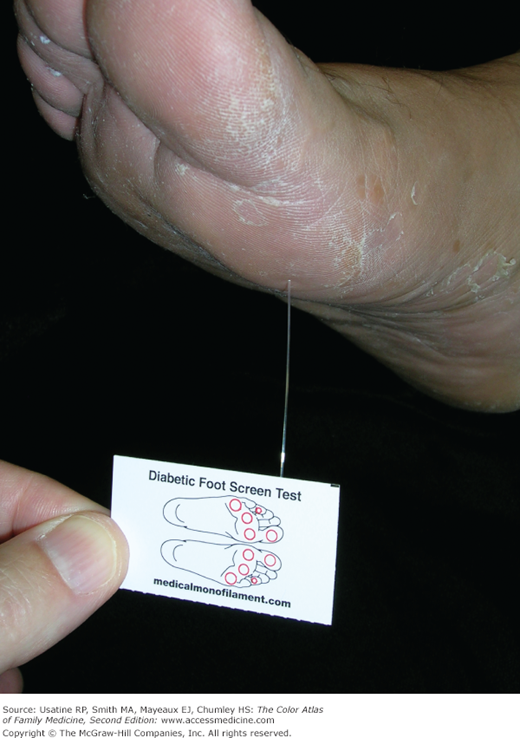
A 66-year-old man with obesity and mild hypertension controlled with a diuretic presents with increasing nocturia and excessive thirst. He has no other urinary symptoms and denies any visual problems. His mother had diabetes and died at age 85 years from a heart attack. His only other concern is a recurrent fungal infection on his feet. His blood pressure in the office today is 135/85 mm Hg and his finger stick blood sugar is 220 mg/dL. You explain that based on his elevated blood sugar, he has diabetes mellitus. Physical exam findings confirm the diagnosis of tinea pedis (Figure 219-1). A monofilament test demonstrates normal sensation in his feet. You order a fasting blood sugar, lipid profile, serum electrolytes, creatinine, and hemoglobin A1C. You ask him to return next week for a more complete examination, review of his test results, and diabetes education. You ask him and his wife to consider meeting with a nutritionist, and briefly review treatment options, including diet, exercise, and metformin, as well as a possible need to improve his blood pressure control or switch to another agent. You suggest a nonprescription antifungal cream and will see if he needs additional treatment for his feet at follow-up. The patient is referred to an ophthalmologist who finds diabetic nonproliferative retinopathy (Figure 219-2).
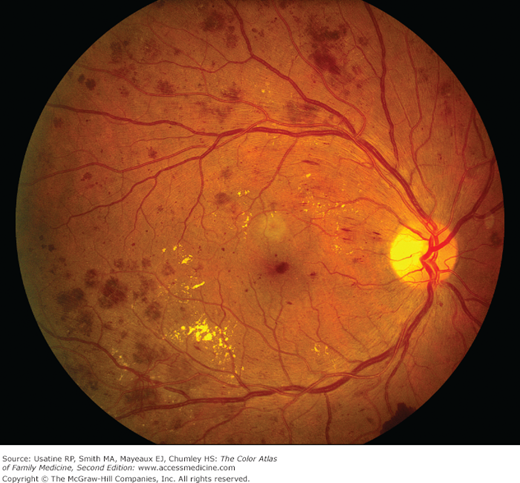
Figure 219-2
Nonproliferative diabetic retinopathy, with scattered intraretinal dot-blot and flame hemorrhages, along with macular exudates. Macular exudates can be related to diabetic macular edema, which accounts for a large portion of poor vision and disability secondary to diabetic retinopathy. (Courtesy of Andrew Sanchez, COA.)
Introduction
Diabetes is a group of disorders caused by a complex interaction between genetic susceptibility, environmental factors, and personal lifestyle choices that share the phenotype of hyperglycemia. Type 2 diabetes mellitus (DM) is a heterogeneous group of chronic disorders caused by a progressive insulin secretory defect and increased glucose production in the setting of insulin resistance.
Epidemiology
- Prevalence—In the United States, 25.8 million adults and children (8.3% of the population), including 18.8 million who have been diagnosed, have diabetes. This includes about 1 in 400 children and adolescents and 26.9% of people age 65 years and older. Type 2 DM is the most common form, accounting for more than 90% of cases.
- Incidence—In the United States in 2010, there were 1.9 million new cases among individuals 19 years of age and older.
- Highest rates of diabetes are in non-Hispanic blacks (12.6%), followed by Hispanics (11.8%), Asian Americans (8.4%), and non-Hispanic whites (7.1%).
- In 2007, total costs of diagnosed DM in the United States were $174 billion ($116 billion in direct medical costs).
Etiology and Pathophysiology
- Insulin resistance is attributed to obesity, inactivity, and genetic factors (including defects in β-cell function and insulin action).
- Initially, the pancreatic β cells increase insulin production to overcome insulin resistance and maintain euglycemia. Eventually, β cells fail, resulting in hyperglycemia.
- Other contributing factors include diseases of the pancreas (e.g., pancreatitis, hemochromatosis), infection (e.g., cytomegalovirus), and other endocrinopathies (e.g., hyperthyroidism [see Chapter 227, Graves Disease and Goiter] and acromegaly [see Chapter 228, Acromegaly]).
- Microvascular and macrovascular diseases may result from hyperglycemia or other metabolic changes.
Risk Factors
- Obesity.*
- Red meat consumption (relative risk [RR] 1.51; 95% confidence interval [CI], 1.25, 1.83 for 50 g processed red meat/day).5
- Physical inactivity (also television viewing 2 h/day; odds ratio [OR] 1.20; 95% CI, 1.14 to 1.27).6*
- Nonwhite race.*
- First-degree relative with diabetes, hypertension, or myocardial infarction.*
- Prior gestational diabetes.*
- Impaired glucose tolerance (hazard ratio [HR] 13.2, 95% CI, 10.8 to 16.2).7
- Polycystic ovarian syndrome.
- Coronary heart disease, hypertension.*
- Smoking (OR [current smoker] 1.44; 95% CI, 1.31 to 1.58).
- Antipsychotic therapy for patients with schizophrenia or severe bipolar disease.*
- Previously identified glucose intolerance.*
- Prolonged use of oral corticosteroids.
Many risk models and scores have been developed to predict the development of DM.8 Authors of a systematic review identified seven risk scores that had high potential for use in practice based on similar components and discriminatory properties.4 One of these, the Framingham Offspring Study,9 uses fasting plasma glucose levels, body mass index, high density lipoprotein cholesterol and triglyceride levels, parental history of diabetes and blood pressure to determine risk. Unfortunately, none of the models were developed on a cohort recruited prospectively, and no studies have demonstrated a reduction in incident DM using risk scoring and intervention.
*Risk factors used by the American Association of Clinical Endocrinologists (AACE) in making decisions for screening patients for DM (see below).4
Diagnosis
The AACE guideline defines the diagnosis of diabetes as a fasting (8 or more hours of no caloric intake) glucose level 126 mg/dL or greater (≥7.0 mmol/L), or a 2-hour plasma glucose 200 mg/dL or greater (≥11.1 mmol/L) on a 75-g oral glucose tolerance test, or a random plasma glucose level of greater than 200 mg/dL with symptoms of diabetes, or a hemoglobin A1C level of 6.5% or higher.4 SOR A The same test should be repeated on a different day to confirm the diagnosis unless the patient has unequivocal hyperglycemia or severe metabolic stress. SOR C
- Many patients with type 2 DM are asymptomatic.
- Patients may report polydipsia, polyuria, and blurred vision.
- Funduscopic examination may reveal signs of retinopathy (hard exudates, hemorrhages) (Figure 219-2; see Chapter 20, Diabetic Retinopathy).
- Patients with diabetic neuropathy may have abnormalities on monofilament, vibration, and superficial pain testing.
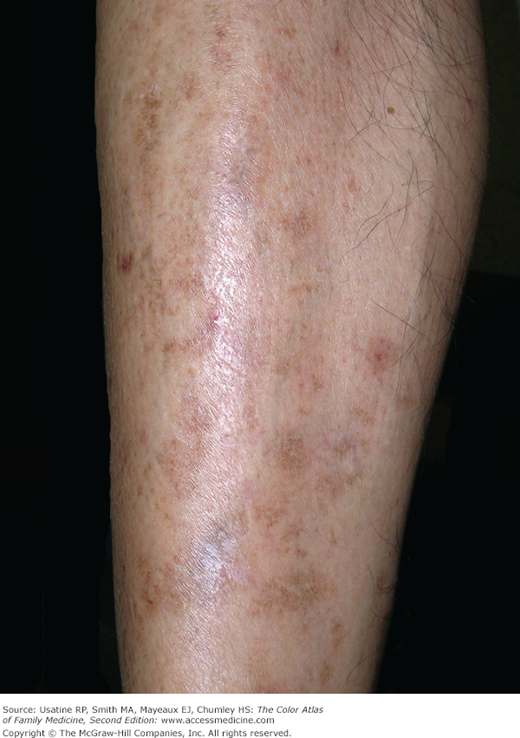
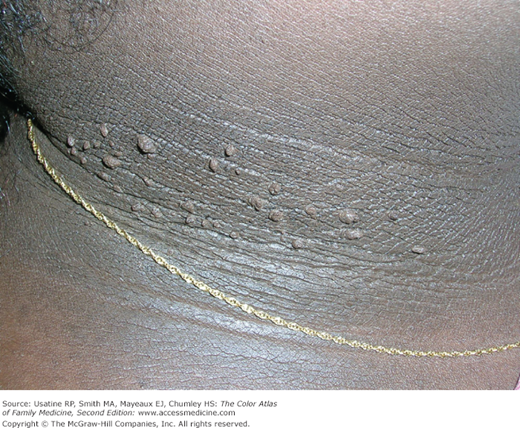
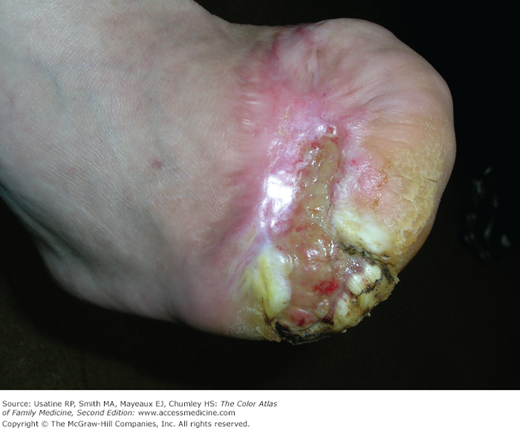
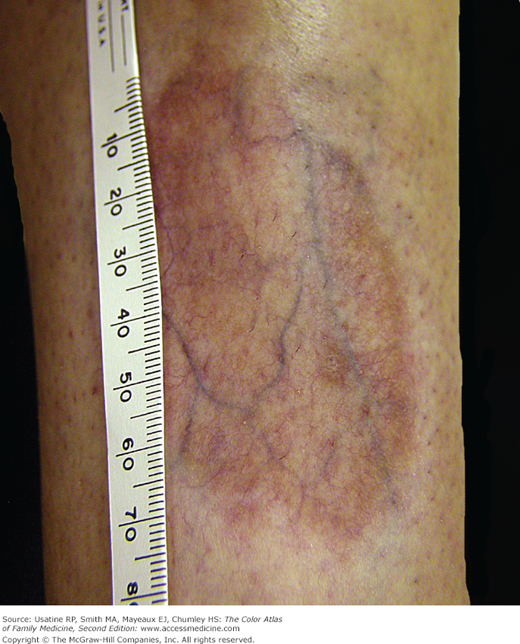
- Skin changes in patients with DM include diabetic dermopathy in 15% to 40% of patients (Figure 219-3; see Chapter 221, Diabetic Dermopathy),10 acanthosis nigricans in approximately one-third of patients (Figure 219-4; see Chapter 220, Acanthosis Nigricans),11 diabetic foot ulcers (Figure 219-5; see Chapter 210, Ischemic Ulcer), and, uncommonly, necrobiosis lipoidica (Figure 219-6; see Chapter 222, Necrobiosis Lipoidica).
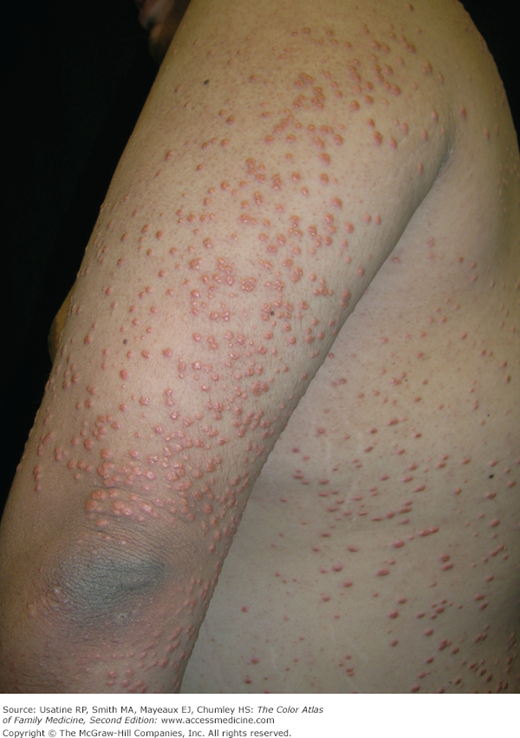
- Hyperlipidemia may result in eruptive xanthomas (Figure 219-7) or xanthelasma.
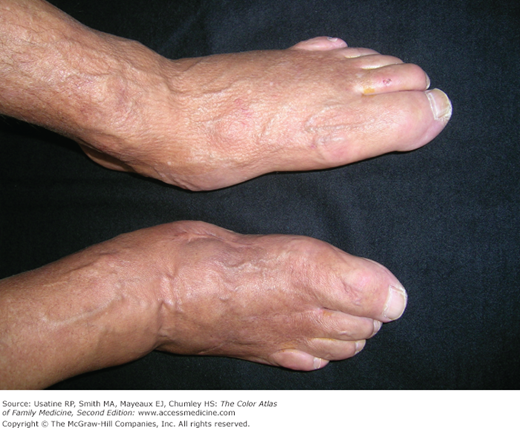
- Patients with DM of prolonged duration may also have Charcot joints (Figure 219-8; see Chapter 212, Charcot Joints).12
- The American Diabetes Association (ADA) recommends fasting plasma glucose as the preferred diagnostic test because of poor patient acceptance of the glucose tolerance test.13 A value of 126 mg/dL or greater is diagnostic, although a second confirming test is recommended. Other tests that can be used for diagnosis are listed above.
- Hemoglobin A1C is less sensitive to low levels of hyperglycemia than either the fasting plasma glucose or glucose tolerance tests and is not currently recommended by the ADA as a diagnostic test.
- A random capillary blood glucose may be a reasonable alternative. Compared with traditional criteria, a capillary blood glucose level of greater than 120 mg/dL has a sensitivity of 75% and specificity of 88% for the diagnosis of type 2 DM.14
- In cases where there is uncertainty regarding the diagnosis of type 1 or type 2 DM, glucagon-stimulated C-peptide, 2-hour postprandial urinary C-peptide-to-creatinine ratio, and 4-hour postprandial urinary C-peptide concentration can be used help distinguish between the types.
Management
The primary treatment goals for the patient with DM are to aggressively control blood pressure (<130/80 mm Hg) and lower lipids (low-density lipoprotein [LDL] goal is <100 mg/dL) to improve cardiovascular and all-cause mortality. Reasonable blood glucose control (hemoglobin A1C near 7%) is usually undertaken with metformin; this author believes that tight/strict control should no longer be stressed as it does not improve most outcomes and increases episodes of hypoglycemia. In the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation) trial (N = 11,140), there were no differences seen in the rates of major macrovascular events or overall mortality between patients randomized to intense control (hemoglobin A1C ≤6.5%) or standard control.15 Furthermore, in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial, intensive insulin therapy in patients with type 2 DM was associated with increased mortality in participants randomized to the very intensive glycemic control arm (hemoglobin A1C <P6%).16 A recent metaanalysis of 14 trials also concluded that intensive control did not reduce all-cause mortality; data were insufficient to confirm a relative risk reduction for cardiovascular morbidity or mortality, composite microvascular complications, or retinopathy at a magnitude of 10%.17 Intensive glycemic control increased the relative risk of severe hypoglycemia by 30%.17</P6%).
In epidemiologic research, a hemoglobin A1C level less than 7% is associated with the best outcomes and the ADA uses a hemoglobin A1C of less than 7% as the benchmark for adequate control. The AACE recommends a glucose target of 6.5% or less in most nonpregnant adults if it can be achieved safely, as near-normal levels may prevent microvascular (e.g., retinopathy) complications.4 SOR C
Diet and exercise interventions:
- Diet—Nutrition advice may be best delivered by a registered dietician familiar with DM. Guidelines from the ADA and AACE agree that the diet should contain carbohydrate from fruits, vegetables, whole grains, legumes, and low-fat milk, and be kept consistent on a day-to-day basis with respect to time and amount.18 In otherwise healthy patients with DM, optimal diets include protein (15% to 20% of daily energy intake), fiber (25 to 50 g/day, with special emphasis on soluble fiber sources [7 to 13 g], to help to lower cholesterol), and dietary fat of less than 30% of daily energy intake.
- A Cochrane review of 11 randomized controlled trials (RCTs) concluded that a low-glycemic index diet improved glycemic control over a higher-glycemic index diet with fewer hypoglycemic episodes in one trial and fewer hyperglycemic episodes in another trial.19
- Daily consumption of vitamin D (500 IU as a fortified yogurt drink or supplement vs. placebo) improved glycemic control in one trial of patients with type 2 DM,20 but not in another trial with patients with type 2 DM and normal vitamin D levels.21 Vitamin D appears to have a positive effect on insulin resistance.22
- Weight-loss strategies for those who are overweight include diet, physical activity, and behavioral interventions. This combination can result in slight sustained weight loss (1.7 kg or 3.1% of baseline body weight) in comparison with usual care and lowers hemoglobin A1C.23 SOR A A more recent RCT (N = 593) found an intensive dietary intervention (dietary consultation every 3 months with monthly nurse support) reduced hemoglobin A1C over the control group (actually had increased hemoglobin A1C); the activity intervention did not confer additional benefit.24
- Even without weight loss, structured exercise (>150 min/wk) significantly improves glycemic control in patients with type 2 DM.25 SOR A In this metaanalysis, a combination of dietary and exercise advice also lowered hemoglobin A1C.25 Individuals with type 2 DM are advised to get 30 to 90 min/day (AACE) or 90 to 150 min/wk (ADA) of exercise to improve glycemic control.7,13
Education, self-management, and self-monitoring interventions:
- Group-based diabetes education is associated with reductions in hemoglobin A1C, systolic blood pressure, body weight, and the need for diabetic medications.26 SOR A In another Cochrane review, use of a specialized diabetes nurse or case manager improved short-term glycemic control but not improve longer-term outcomes, such as hospital admission rates and quality of life.27 Although one recent trial (N = 201) of behavioral support (video and five telephone sessions) for patients with poorly controlled DM did not show benefit on outcomes,28 another trial (N = 222) using five educator-led group sessions for patients with poorly controlled DM did find improved glycemic control over the control group, although there were no differences in quality of life or frequency of diabetes self-care.29
- Authors of a Cochrane review of 12 trials on blood glucose self-monitoring in patients with type 2 DM not using insulin found a small effect of self-monitoring on glycemic control (reduced hemoglobin A1C) that lasted up to 6 months after initiation but not at or beyond 12 months.30 There was no evidence that self-monitoring affected patient satisfaction, general well-being, or general health-related quality of life. However, self-monitoring of glucose may be useful for assessing and preventing hypoglycemia and adjusting medications, medical nutrition therapy, and physical activity.4
- Structured goal setting was shown in a RCT (N = 87) to improve glycemic control up to 1-year postintervention.31
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree