Figure 52.1. Figure showing the relationship between updated mean HbA1c levels and complications for retinopathy and microalbuminuria.
SOURCE: From Diabetes Control and Complications Trial Research Group. The absence of a glycemic threshold for the development of long-term complications. Diabetes 45: 1289–1298, 1996.
The United Kingdom Prospective Diabetes Study (UKPDS) studied patients with newly diagnosed type 2 diabetes to determine whether intensive therapy was superior to conventional (dietary) intervention. The UKPDS had two glycemic substudies. In the first, 3876 subjects (mean age 54) were randomly assigned to intensive control with sulfonylurea or insulin versus conventional control. In the second, 753 obese patients (mean age 53) were randomly assigned to metformin versus conventional control. The intensive strategy aimed for a goal fasting glucose of <108 mg/dL. Conventional control was pursued with diet, and medications were added for symptoms of hyperglycemia or fasting plasma glucose >270 mg/dL. The majority of the conventional group crossed over to the treatments given in the intensive groups, and more than 20% of the intensive groups had additional medications added, complicating interpretation of the results. Nonetheless, the intensive group maintained a mean HbA1c of 7% compared to 7.9% in the conventional group. Intention-to-treat analyses showed a 12% reduction in aggregate diabetes outcomes, which was largely due to a 25% reduction in treatment for retinopathy. The intensive group gained 2.9 kg more, on average, and had more hypoglycemia. In the metformin cohort, the intensive group maintained a mean HbA1c of 7.4% compared to 8% in the control group. Intention-to-treat analysis showed that patients assigned to the metformin group had significant 32% risk reduction for any diabetes-related endpoint and a 36% reduction in all-cause mortality. Ten-year follow-up of this cohort showed persistent benefits in any diabetes-related endpoint as well as lower rates of myocardial infarction and death in both intensively treated groups. UKPDS also included a factorial design blood pressure study and analyses of the results have yielded further information on the role of blood pressure control in contributing to reduction in complications, as outlined below.
The Kumamoto trial in Japanese patients with type 2 diabetes, modeled after the DCCT, randomly assigned 110 patients to intensive control with multiple insulin injections, achieving a mean HbA1c of 7.1%, or to conventional insulin treatment, associated with a mean HbA1c of 9.4%, over the 6 years of the study. Intensive treatment was associated with significant reductions in retinopathy, neuropathy, and nephropathy, similar in magnitude to the results in the DCCT.
EFFECT OF GLYCEMIC CONTROL ON PRESERVING PANCREATIC FUNCTION
In addition to reducing the long-term complications of diabetes, better glycemic control may improve and potentially preserve beta-cell function, which in turn sustains lower glycemic control and reduces complications. Acutely, glucose and free fatty acids stimulate insulin secretion. However, chronically elevated levels of glucose and free fatty acids desensitize and may poison the beta cell. Early in the course of type 1 diabetes and during type 2 diabetes, residual beta-cell function can be improved by reducing high ambient glucose and free fatty acid levels, which adversely affect beta-cell function through “glucotoxicity” and lipotoxicity, respectively. The reduction of glucotoxicity and lipotoxicity with resultant improvement in beta-cell function has been achieved with intensive insulin treatment in type 1 and type 2 diabetes. In type 2 diabetes, reduction of glucotoxicity may also be accomplished by other pharmacologic (non-insulin drugs) or nonpharmacologic (diet, exercise) means. In addition to contributing to improved glycemia and reduced long-term complications, preserved beta-cell function promotes hypoglycemia awareness, allowing the safe application of intensive therapy.
RETINOPATHY
In the United States, diabetic retinopathy is the leading cause of vision loss among adults aged 20–74, with 12,000–24,000 new cases of blindness annually and with 28.5% of all adults with diabetes reporting some retinopathy and 4.4% reporting advanced retinopathy. Diabetic retinopathy is largely related to the duration of diabetes, the degree of glycemic control, and hypertension and dyslipidemia. Glaucoma and cataracts are more common in diabetes and also contribute to loss of vision.
SCREENING
Because retinopathy is estimated to develop only after 5 years of hyperglycemia, and the onset of hyperglycemia is usually easily recognized in type 1 diabetes, patients with type 1 diabetes need be screened with a dilated fundoscopic examination 5 years after diagnosis. Patients with type 2 diabetes, who frequently have an indolent onset of diabetes and may therefore have unrecognized prior diabetes and complications at the time of diagnosis, should be screened for retinopathy at the time of diabetes diagnosis. Current recommendations suggest, in general, annual screening thereafter.
PREVENTION
Glycemic control has been shown to reduce the risk of development and progression of retinopathy; every 1% reduction in HbA1c is associated with an approximately 40% reduction in retinopathy in both type 1 (DCCT) and type 2 diabetes (UKPDS; see Figure 52.2). Blood pressure control reduces the risk of progression of retinopathy in type 2 diabetes. Lowering triglyceride levels reduces the risk of retinopathy in type 2 diabetes. Notably, intensification of glycemic control in patients with previously poorly controlled diabetes and preexisting retinopathy can precipitate worsening retinopathy, at least transiently; close monitoring by an ophthalmologist is warranted in such patients. Efforts at screening and more intensive interventions correspond to a decrease in the prevalence of visual impairment from diabetes from 26% in 1997 to 21–22% in 2005.
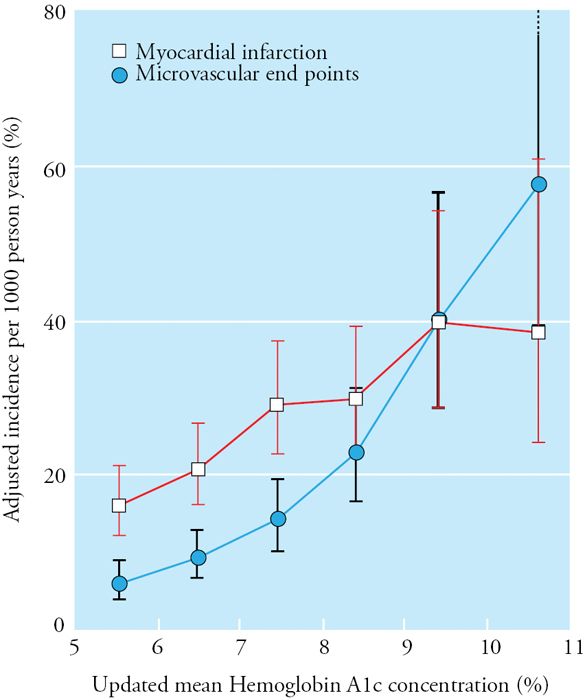
Figure 52.2. Relationship between mean HbA1c and microvascular and macrovascular complications in the UKPDS. SOURCE: Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412.
PATHOPHYSIOLOGY AND TREATMENT
Diabetic retinopathy occurs in two forms: nonproliferative retinopathy, characterized by abnormal microvasculature resulting in leakage of serum proteins and manifested as microaneurysms and hard exudates, and the more advanced preproliferative and proliferative retinopathy. Preproliferative retinopathy is caused by occlusion of the small vessels leading to retinal ischemia with retinal infarcts, manifested as soft “cotton wool” exudates. Retinal ischemia promotes the secretion of growth factors such as vascular endothelial growth factor (VEGF), which in turn trigger “neovascularization”—proliferation of friable vessels in the fundus and into the vitreous.
Loss of vision in diabetic retinopathy is due to one or a combination of the following: macular edema resulting from leaking capillaries near the maculae, macular ischemia from obstructed vessels supplying the maculae, retinal detachment related to traction from proliferative retinopathy vessels and fibroproliferative scarring, vitreous hemorrhage, and neovascular glaucoma. Primary prevention for diabetic neuropathy is supported by type A evidence. Treatment targets blood pressure and glycemia.
Laser photocoagulation therapy is used to treat macular edema and proliferative retinopathy, according to indications established by the Diabetic Retinopathy Study and Early Treatment Diabetic Retinopathy Study. Severe nonproliferative diabetic retinopathy (with intraretinal hemorrhages in all four quadrants of the eye) and/or proliferative diabetic retinopathy (with neovascularization of the optic disc or neovascularization of a significant portion of the retina elsewhere) are treated with panretinal photocoagulation, which reduces the risk of severe vision loss from 15.9% to 6.4% (>50% reduction). This treatment sacrifices the damaged portion of the retina outside of the macula to decrease the ischemic stimulus to neovascularization. Intraocular steroids or anti-VEGF are noninferior and may be superior for some forms of PDR. Clinically significant macular edema, which is difficult to appreciate on direct ophthalmoscopy, is treated with focal photocoagulation or intraocular anti-VEGF therapy (ranibizumab or bevacizumab, which reduces the risk of progression of visual loss in macular edema from 20% to 8%. Aspirin (for prophylaxis of cardiovascular disease) is not contraindicated in the presence of retinopathy but does not appear to reduce retinopathy.
NEPHROPATHY
Diabetic nephropathy is the leading cause of end-stage renal disease (ESRD) in the United States, accounting for 44% of all new cases of kidney failure (about 48,374 people beginning treatment for ESRD due to diabetes) in 2008. Twenty to thirty percent of patients with diabetes develop diabetic nephropathy, with nonwhite groups having a higher rate of progression. The rate of increase in new cases and the frequency of progression to ESRD have declined since 1997, probably owing to intensive treatment of hyperglycemia and hypertension.
The earliest sign of diabetic nephropathy is an elevated level of urinary albumin excretion called microalbuminuria, which can be detected on a spot urine collection and represents 30 to 300 mg of albumin excretion per day. Conventional urine dipstick testing only detects levels >300 mg/day, usually called macroalbuminuria or clinical proteinuria. Presence of microalbuminuria is correlated not only with risk for progression to more severe kidney dysfunction, but also with high cardiovascular disease risk in patients with and without diabetes. Thirty percent of patients with type 1 diabetes have microalbuminuria after 15 years of diabetes. Half of this population progresses to overt nephropathy characterized by macro albuminuria (>300 mg/24 hours). The development of microalbuminuria late in the course of diabetes corresponds to a lower risk of progression to nephropathy. Twenty-five to forty percent of patients with type 2 diabetes have microalbuminuria after 10–15 years of diabetes, and approximately one-half of these will progress to overt nephropathy. Risk of progression is increased in African Americans and with hyperglycemia, hypertension, and smoking. Overt nephropathy almost always progresses to decreasing glomerular filtration rate and, given enough time, end stage renal disease (ESRD). Although most patients with diabetes who develop severe kidney dysfunction progress from albuminuria to reduced GFR, in some patients albuminuria precedes a decline in GFR. The absence of albuminuria should raise concern that a cause other than diabetes may underlie the kidney disease.
SCREENING
Screening for diabetic nephropathy includes an annual test for urine microalbumin excretion in a spot urine sample in which albumin in milligrams is standardized to grams of urine creatinine. This ratio roughly approximates the daily albumin excretion in milligrams per day. Less than 30 mg albumin per gram of creatinine is considered normal (although normal levels of albuminuria are probably closer to 10 mg per gram of creatinine). Vigorous exercise, acute hyperglycemia, fever, infection, congestive heart failure (CHF), severe hypertension, and low muscle mass can falsely elevate the degree of microalbuminuria, so a positive screen should be confirmed with two of three midmorning samples. In addition, serum creatinine should be measured yearly to estimate glomerular filtration rate and stage the degree of chronic kidney disease (CKD). Referral to nephrologist is recommended as stage 3 CKD develops (eGFR <60 mL/min; see table 52.2).
Table 52.2 SUMMARY OF REFERRALS FOR ADULTS WITH DIABETES
| Dilated fundoscopic | |
| examination | 5 years after diagnosis, then annually |
| Type 1 diabetes | Annually |
| Type 2 diabetes | |
| Podiatry | When severe neuropathy is present |
| Dentistry | Annually |
| Certified diabetes | At diagnosis, and as indicated |
| educator for diabetes | |
| self-management education | |
| Registered dietician for medical nutrition therapy | At diagnosis, and as indicated |
| Mental health professional | As indicated |
PREVENTION
Optimizing glycemia has been shown to prevent development and slow the rate of progression of nephropathy in both type 1 and type 2 diabetes. Maintaining blood pressure at less than 140/80 mm Hg also prevents development and progression of proteinuric kidney disease, has been shown to have additive benefit to glycemic control in patients with type 2 diabetes, and likely has the same effect in type 1 diabetes. Both are ADA evidence level A recommendations.
TREATMENT
When microalbuminuria develops (i.e., >30 μg albumin/mg creatinine), angiotensin-converting enzyme inhibitors (ACEi) have been shown to prevent progression of nephropathy in patients with type 1 diabetes, and both ACEi and angiotensin receptor blockers (ARB) have been shown to prevent progression in patients with type 2 diabetes. The dose of the ACEi or ARB should be maximized in patients with microalbuminuria or nephropathy, monitoring for hyperkalemia, elevation of creatinine, and other side effects. The ACEi should be initiated first based on its lower cost; if the ACEi is not tolerated, an ARB can be substituted.
NEUROPATHY AND LOWER EXTREMITY DISEASE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


