Chapter 53 ∗Removed from market 2011 but available by special FDA permission. • First-degree relative with diabetes • High-risk race/ethnicity (e.g., African American, Latino, Native American, Asian American, Pacific Islander) • A1c >5.7%, impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) • History of gestational diabetes mellitus (GDM) or delivery of a baby weighing >9 pounds • Hypertension ≥140/90 mm Hg in adults or on therapy for hypertension • HDL cholesterol ≤35 mg/dl and/or triglyceride level ≥250 mg/dl • Women with polycystic ovary syndrome (PCOS) • Other clinical conditions associated with insulin resistance (e.g., severe obesity or acanthosis nigricans) • History should include questions about skin changes, visual problems (e.g., history of retinopathy, date of last ophthalmologic examination), thyroid function (e.g., history of hypothyroid or hyperthyroid disease), cardiac arrhythmias, hypertension, lipid abnormalities, history of CAD and/or CHF, and hematologic, pulmonary, GI (e.g., chronic diarrhea, constipation, early satiety, liver disease), GU (e.g., urinary tract infection, difficulty voiding, incontinence, erectile dysfunction), obstetric and gynecologic (e.g., history of gestational diabetes, anticipation of pregnancy, contraceptive use), neurologic (e.g., weakness, muscle wasting, paresthesias, hyperesthesia, hypoglycemic unawareness), and vascular problems (e.g., leg pain, transient ischemic attacks). • Physical examination should include assessment of height, weight, BMI, BP, vital signs, eye, mouth, thyroid, heart, lungs, abdomen (e.g., hepatic enlargement), bruits, pulses, hands, feet, skin (e.g., acanthosis nigricans, inflammation, infection), and nervous system (e.g., reflexes, sensory examination of feet). • Laboratory evaluation includes CBC, CMP (including creatinine, BUN, TSH, Hbg, and HbA1c), fasting lipid profile, urinalysis, test for microalbuminuria, and ECG in adults. The HbA1c reflects the state of glycemia for the past 8 to 12 weeks. See Tables 53-1 and 53-2. TABLE 53-1 ADA-Recommended Glycemic Goals TABLE 53-2 Mechanisms of Action of Diabetic Medications • Suppress glucagon production, especially in the postprandial state • Reduce postprandial hepatic glucose production • Centrally mediate induction of satiety Generally accepted guidelines for managing diabetes have been established by the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (ACE). Although subtle differences are evident, most guidelines are similar. See http://care.diabetesjournals.org/content/35/Supplement_1/S11.full.pdf+html and http://care.diabetesjournals.org/content/35/3/660.1.full. • Educational interventions are likely to be beneficial. • Metformin is more effective than diet alone. • Sulfonylureas reduce A1c further compared with placebo or diet alone. • New sulfonylureas (second generation) are better than older sulfonylureas (first generation) in contributing to hypoglycemia. • To prevent or delay onset of diabetes, patients with impaired glucose tolerance (IGT) or IFG should be advised to lose 5% to 10% of body weight and to increase physical activity to at least 150 minutes per week of moderate activity such as walking. Follow-up counseling seems to improve the likelihood of success. • Metformin therapy should be considered in patients who are at very high risk for diabetes, based on combined IFG and IGT, plus other risk factors, and who are obese and younger than 60 years of age. • Studies show that intensive glucose control (A1c 6.5% to 7%) improves mortality and morbidity and reduces costs. • Because lowering A1c levels to an average of about 7% has been shown to reduce microvascular and neuropathic complications of diabetes, the target A1c goal for nonpregnant women is generally less than 7% (less than 6.5% per AACE guidelines). However, A1c and other treatment goals should be individualized. • For selected individual patients, the A1c goal is as close to normal (<6%) as possible without significant hypoglycemia, in light of epidemiologic studies showing a small but incremental benefit to lowering A1c from 7% into the normal range. • Because of the progressive nature of diabetes, practitioners should expect to titrate medications and augment therapy over time. • For children, patients with a history of severe hypoglycemia, those with limited life expectancies, individuals with comorbid conditions, and those with long duration of diabetes and minimal or stable microvascular complications, less stringent A1c goals may be appropriate. • As glucotoxicity and further β-cell dysfunction occur, the patient most likely will require higher doses of medications or the addition of new agents to maintain HbA1c at goal. • Monitoring carbohydrate intake is essential to achieving glycemic control, whether by carbohydrate counting, exchanges, or experience-based estimation. For patients with diabetes, glycemic index and glycemic load use may modestly improve glycemic control vs. that observed when considering only total carbohydrate. • People with diabetes should perform at least 150 minutes per week of moderate-intensity aerobic physical activity (50% to 70% of maximum heart rate), and unless there are contraindications, those with type 2 diabetes should perform resistance training three times per week. • Goals of care must be established with the patient and should reflect the individualized plan of care specifically designed for that patient. The plan of care should account for costs, side effects, preferences, and A1c goals. • Evidence shows that maintaining individualized glycemic goals should be followed in treatment for the elderly. Importance is placed on maintaining optimal control to lessen the vascular and neurologic complications of the disease. The first step is to establish glycemic goals for the individual patient. The patient’s age, ability to provide self-care, other medical problems, social support, and financial issues all play a role in decisions regarding individual glycemic goals. (See Table 53-1 for ADA-recommended glycemic goals.) Many patients with diabetes resist taking medications and may rely on herbal products to control symptoms. Some of these herbal products have potential interactions with other medications that the patient may be taking. (See Table 53-9 for a list of potential drug interactions.)
Diabetes Mellitus Agents
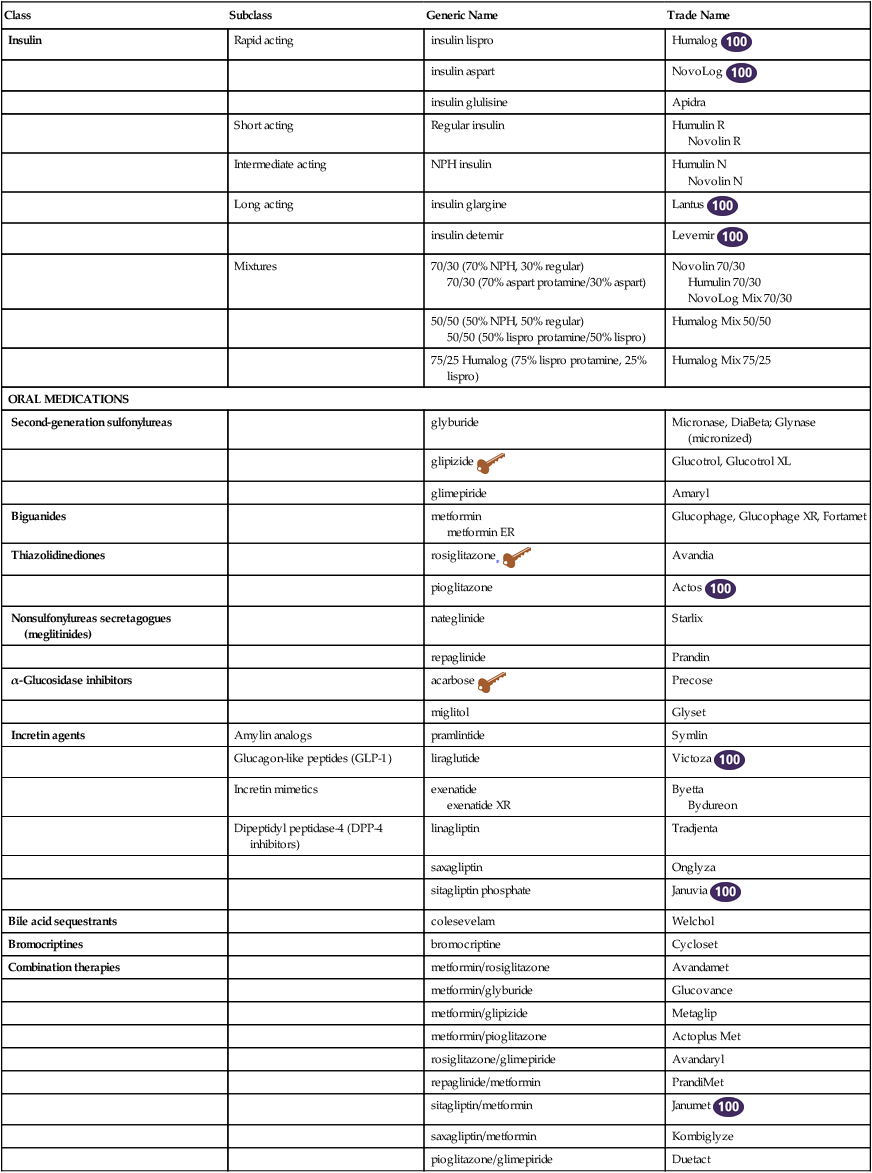
Class
Subclass
Generic Name
Trade Name
Insulin
Rapid acting
insulin lispro
Humalog ![]()
insulin aspart
NovoLog ![]()
insulin glulisine
Apidra
Short acting
Regular insulin
Humulin R
Novolin R
Intermediate acting
NPH insulin
Humulin N
Novolin N
Long acting
insulin glargine
Lantus ![]()
insulin detemir
Levemir ![]()
Mixtures
70/30 (70% NPH, 30% regular)
70/30 (70% aspart protamine/30% aspart)
Novolin 70/30
Humulin 70/30
NovoLog Mix 70/30
50/50 (50% NPH, 50% regular)
50/50 (50% lispro protamine/50% lispro)
Humalog Mix 50/50
75/25 Humalog (75% lispro protamine, 25% lispro)
Humalog Mix 75/25
ORAL MEDICATIONS
Second-generation sulfonylureas
glyburide
Micronase, DiaBeta; Glynase (micronized)
glipizide ![]()
Glucotrol, Glucotrol XL
glimepiride
Amaryl
Biguanides
metformin
metformin ER
Glucophage, Glucophage XR, Fortamet
Thiazolidinediones
rosiglitazone∗ ![]()
Avandia
pioglitazone
Actos ![]()
Nonsulfonylureas secretagogues (meglitinides)
nateglinide
Starlix
repaglinide
Prandin
α-Glucosidase inhibitors
acarbose ![]()
Precose
miglitol
Glyset
Incretin agents
Amylin analogs
pramlintide
Symlin
Glucagon-like peptides (GLP-1)
liraglutide
Victoza ![]()
Incretin mimetics
exenatide
exenatide XR
Byetta
Bydureon
Dipeptidyl peptidase-4 (DPP-4 inhibitors)
linagliptin
Tradjenta
saxagliptin
Onglyza
sitagliptin phosphate
Januvia ![]()
Bile acid sequestrants
colesevelam
Welchol
Bromocriptines
bromocriptine
Cycloset
Combination therapies
metformin/rosiglitazone
Avandamet
metformin/glyburide
Glucovance
metformin/glipizide
Metaglip
metformin/pioglitazone
Actoplus Met
rosiglitazone/glimepiride
Avandaryl
repaglinide/metformin
PrandiMet
sitagliptin/metformin
Janumet ![]()
saxagliptin/metformin
Kombiglyze
pioglitazone/glimepiride
Duetact
Therapeutic Overview
Anatomy and Physiology
Disease Process
Assessment
Mechanism of Action
Normal
Goal
Additional Action Suggested
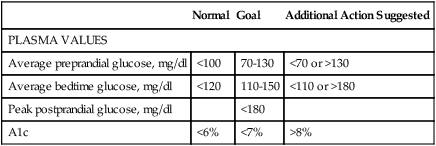
PLASMA VALUES
Average preprandial glucose, mg/dl
<100
70-130
<70 or >130
Average bedtime glucose, mg/dl
<120
110-150
<110 or >180
Peak postprandial glucose, mg/dl
<180
A1c
<6%
<7%
>8%
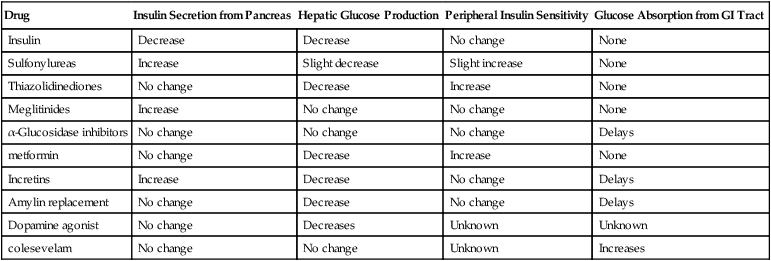
Drug
Insulin Secretion from Pancreas
Hepatic Glucose Production
Peripheral Insulin Sensitivity
Glucose Absorption from GI Tract
Insulin
Decrease
Decrease
No change
None
Sulfonylureas
Increase
Slight decrease
Slight increase
None
Thiazolidinediones
No change
Decrease
Increase
None
Meglitinides
Increase
No change
No change
None
α-Glucosidase inhibitors
No change
No change
No change
Delays
metformin
No change
Decrease
Increase
None
Incretins
Increase
Decrease
No change
Delays
Amylin replacement
No change
Decrease
No change
Delays
Dopamine agonist
No change
Decreases
Unknown
Unknown
colesevelam
No change
No change
Unknown
Increases
Incretin Agents
Amylin Replacement
Treatment Principles
Standardized Guidelines
Evidence-Based Recommendations
Cardinal Points of Treatment
Nonpharmacologic Treatment
Establish Goals
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


