http://evolve.elsevier.com/Edmunds/NP/
Therapeutic Overview
The pancreas is a gland with both endocrine and exocrine functions. The islets of Langerhans, which constitute only 1% to 2% of the gland, contain more than 1 million cells. Eighty percent of these cells are β-cells that produce and secrete insulin. α-Cells, also a component of the islets, produce glucagon, a potent hormone that promotes glycogenolysis and gluconeogenesis in the liver. The pancreas and the liver are the primary organs of glucose regulation attained via a negative feedback mechanism. When blood glucose rises, β-cells are stimulated by elevated glucose to release insulin. Insulin allows the muscle and the liver to use glucose and to store it as glycogen in the liver. Insulin also facilitates fat storage in adipose tissue, as well as uptake and conversion of amino acids to protein. As blood glucose levels fall, the cells are stimulated to release glucagon, resulting in glycogenolysis and gluconeogenesis in the liver. Glycogenolysis (conversion of glycogen into glucose) and gluconeogenesis (production of glucose from lactate and amino acids) result in increased serum glucose levels.
Insulin lowers blood glucose by enhancing glucose transport via facilitated diffusion into target tissues. Insulin binds to and stimulates receptors on each cell; this in turn fosters transport of glucose through the cell wall. Tissue insensitivity to insulin can occur when defects in receptors or defects in receptor response to insulin are present. Insulin also inhibits lipoprotein lipase, thereby preventing the release of fatty acids into the blood. Insulin promotes the transport and storage of glucose as triglycerides in fat cells.
Pathophysiology
The onset of diabetes involves a relative or absolute lack of insulin and/or insulin resistance and impaired or insufficient target cell receptors. These effects cause a lack of available glucose for cellular metabolism, resulting in glycogenolysis, lipolysis, and gluconeogenesis. Glucose uptake by the liver is impaired with a resultant increase in circulating glucagon. Protein storage is decreased. Overproduction of free fatty acids by fat cells has been noted. Elevated plasma free fatty acid levels increase hepatic glucose production by stimulating gluconeogenesis.
Type 2 diabetes is characterized by two phenomena: insulin resistance and β-cell dysfunction. The cause of type 2 diabetes is unknown, but certain factors increase the risk of development of the disease. With type 2 diabetes, secretion is impaired, hepatic glucose production is increased, and insensitivity to insulin in the tissues (i.e., insulin resistance) occurs.
Initially in type 2 diabetes, tissues become insensitive or resistant to insulin. As a compensatory mechanism, insulin release is increased so that normal glucose levels can be maintained. In fact, often, those in whom type 2 diabetes is newly diagnosed exhibit elevated insulin levels in an attempt to overcome resistance. Type 2 diabetes is a progressive disease that displays further deterioration of β-cell function over time, even when management is optimized. At diagnosis, approximately 50% of β-cell function has been lost.
Insulin secretion occurs in a biphasic manner. The first phase occurs rapidly, peaks in 3 to 5 minutes, and lasts about 10 minutes. This phase is triggered by a meal or a glucose challenge. If the blood glucose concentration remains elevated, then the second phase of insulin is triggered. This involves a slow release of insulin. Persons with type 2 diabetes lose first-phase insulin release, and postprandial hyperglycemia is the first sign. As the body endures continued exposure to hyperglycemia, the β-cells become more dysfunctional; this is referred to as glucotoxicity. This process often begins years prior to diagnosis of type 2 diabetes. With now a relative lack of insulin, gluconeogenesis and glycogenolysis are increased in the liver because these processes are dependent not on levels of glucose but on insulin levels. This further contributes to hyperglycemia and is often responsible for elevated fasting glucose levels.
Autoimmune destruction of β-cells is implicated in the diagnosis of type 1 diabetes. Environmental and genetic predisposition also may play a role. Onset of type 1 diabetes may occur suddenly as with the onset of diabetic ketoacidosis, or slowly with less risk of ketoacidosis as in the case of latent autoimmune diabetes of adults (LADA).
Disease Process
Testing for type 2 diabetes should be considered in all adults who are overweight (BMI ≥25 kg/m2) who have one or more risk factors. In the absence of risk factors, screening should begin at 45 years and older. Testing should be repeated every 3 years. According to the ADA, risk factors for the development of diabetes that should alert the provider to screen for diabetes include the following:
History of Vascular Disease
The Expert Committee on the Diagnosis and Classification of Diabetes under the sponsorship of the ADA modified criteria for diagnosis in 2012. These criteria were adopted by the ADA and represent current guidelines for diagnosis (Box 53-1).
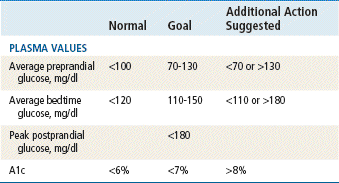
Complications of diabetes include microvascular disease (e.g., nephropathy, retinopathy), macrovascular disease (e.g., coronary artery disease, peripheral vascular disease, cerebrovascular disease, neuropathy), and neuropathic disease (e.g., autonomic and peripheral). Vascular and neuropathic disease contributes to the increased risk of amputation. Multiple studies, such as the Diabetes Mellitus Control and Complications Trial research group (DCCT) and the United Kingdom Prospective Diabetes Study (UKPDS), continue to show that intensive glucose control at or below glycosylated hemoglobin (A1c) of 7% may prevent and/or delay the onset of complications.
Assessment
Conducting a thorough history and physical examination enables the provider to obtain a baseline evaluation, identify early effects and complications of diabetes, and, together with the patient, determine individual glycemic goals. ADA clinical practice recommendations list the following:
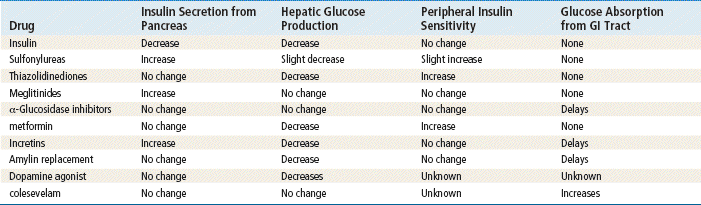
Mechanism of Action
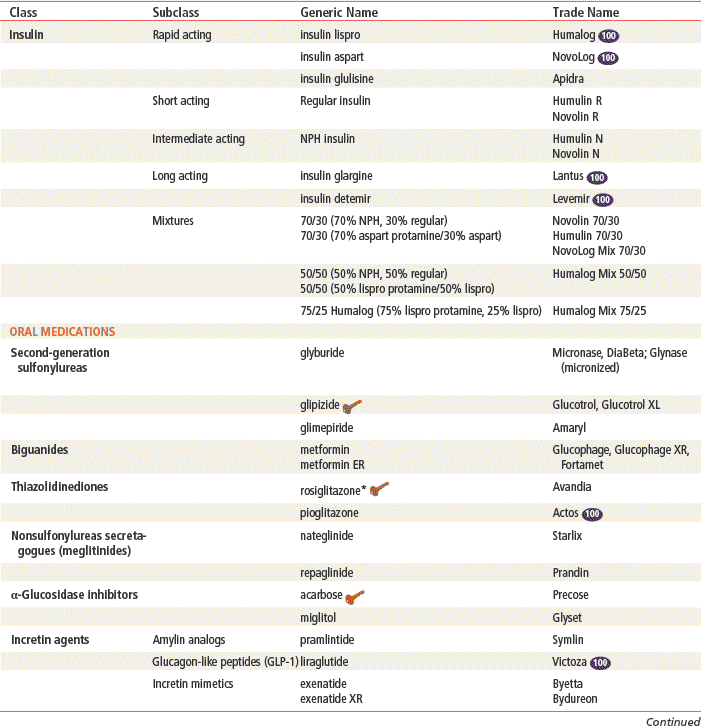
See Tables 53-1 and 53-2.
Insulin
Insulins are proteins that bind to cell wall receptors to allow cellular utilization of glucose. Insulin lowers blood glucose levels by stimulating peripheral glucose uptake, particularly in skeletal muscle and fat, and by inhibiting hepatic glucose production. An adequate supply of insulin is needed for transport of glucose across the cell membrane to sustain life.
Most insulin used today is produced by deoxyribonucleic acid (DNA) recombinant technology and is synthesized in a nonpathogenic strain of Escherichia coli bacteria or Saccharomyces cerevisiae fungus. Advantages of using synthetic human insulin include a decrease in the production of insulin antibodies and a diminished risk for the development of lipodystrophy at the injection site.
Insulin analogs are insulin preparations that are produced by modifying the structure of human insulin. Changing human insulin properties with amino acid substitutions improves the pharmacokinetic profile for optimal physiologic insulin replacement.
Sulfonylureas
Because first-generation sulfonylureas are no longer used, this chapter refers to second-generation sulfonylureas simply as sulfonylureas. (Chlorpropamide is still available in the United States but is rarely used.) Sulfonylureas enhance insulin secretion primarily by binding to receptor sites on β-cells. This causes a decrease in potassium permeability and membrane depolarization. The subsequent increase in intracellular calcium ions causes exocytosis of insulin from secretory granules. Other results include suppression of hepatic glucose production through entry of insulin into the portal vein and increased muscle glucose uptake via elevated insulin levels.
Biguanides
Metformin primarily decreases hepatic glucose production. It also has minor effects on insulin sensitivity in both the liver and peripheral tissues. It has no direct effect on the pancreas and therefore does not enhance insulin secretion. Metformin also has been shown to decrease triglycerides and LDLs and to increase HDLs.
Thiazolidinediones
Rosiglitazone and pioglitazone increase sensitivity in the muscle and liver by improving control of glycemic utilization. This in turn reduces circulating insulin levels. Functioning β-cells are required for these medications to work. Specifically, these drugs are agonists for peroxisome proliferator–activated receptors-γ (PPAR-γ), which are found in adipose tissue, skeletal muscle, and liver. Activation of PPAR-γ receptors regulates the insulin-responsive genes involved in the control of glucose production, transport, and utilization and facilitates the regulation of fatty acid metabolism.
Rosiglitazone and all products containing rosiglitazone have been required to undergo a risk evaluation and mitigation strategy (REMS) so that products are only available by restricted access and distribution system due to the increased risk for heart attacks. Pioglitazone and all pioglitazone containing products now bear warnings about a possible risk of bladder cancer associated with use for more than 1 year.
Meglitinides
Repaglinide and nateglinide lower blood sugar by stimulating the release of insulin from the pancreas in short bursts. The patient must have functioning β-cells for the medication to work. They bind receptor sites to close ATP-dependent potassium channels in the β-cell membrane. This leads to opening of calcium channels, which causes an influx of calcium that induces insulin secretion.
α-Glucosidase Inhibitors
Acarbose and miglitol act through inhibition of pancreatic α-amylase and membrane-bound intestinal α-glucoside hydrolase enzymes. These enzymes are responsible for metabolizing complex starches to oligosaccharides and for breaking down remaining saccharides to glucose and other monosaccharides. This enzyme inhibition delays glucose absorption and lowers postprandial hyperglycemia. These enzymes do not enhance insulin secretion.
Incretin Agents
Pramlintide is an analog of amylin; it is an endogenous peptide that is secreted in conjunction with insulin by the pancreatic β-cells. Pramlintide produces the same physiologic effects as are caused by amylin, but it is stable enough to be used as a medication. Amylin is known to do the following:
Pramlintide is indicated in patients with type 1 diabetes and in those with insulin-requiring type 2 diabetes. It is given by subcutaneous injection three times a day before meals. This agent must be administered as a separate subcutaneous injection but in conjunction with insulin. It is helpful for patients with wide glycemic swings. It is weight neutral or may cause weight loss. The risk of hypoglycemia is significant, and close monitoring of blood glucose followed by frequent adjustments in the dosages of other diabetes medications is required. Its ability to induce weight loss makes it an attractive option for overweight patients. Other adverse reactions include nausea, anorexia, early satiety, and vomiting.
Glucagon-Like Peptide-1 (GLP-1)
Incretins are hormones that are released from the gut postprandially; they often are found in low concentrations in persons with type 2 diabetes. The incretin that has received the most attention is GLP-1. Incretins stimulate insulin secretion in pancreatic β-cells and have been shown to restore both phases of insulin release. GLP-1 regulates glucose homeostasis via multiple complementary actions and along with other incretins are known to do the following:
Exenatide (Byetta and Bydureon)
This agent is derived from a component of the saliva of the Gila monster lizard and has been approved as adjunctive therapy for type 2 diabetes. Exenatide binds to GLP-1 receptors and stimulates insulin secretion when blood sugar is high. It is the first drug that has been shown to restore first-phase insulin secretion, which does not occur in persons with type 2 diabetes. This metabolic defect is responsible for postprandial hyperglycemia. Exenatide also acts to stimulate β-cell replication and neogenesis, increase β-cell mass, and improve glucose tolerance. It is given as an injection before the morning and evening meals. Adverse effects of exenatide include nausea, vomiting, diarrhea, and upper respiratory symptoms. It may cause a reduction in food intake that will necessitate adjustment of the patient’s other diabetes drugs to prevent hypoglycemia. The risk of hypoglycemia is not increased when used with metformin, but this risk is increased when used with sulfonylureas. Exenatide is clearly associated with weight loss.
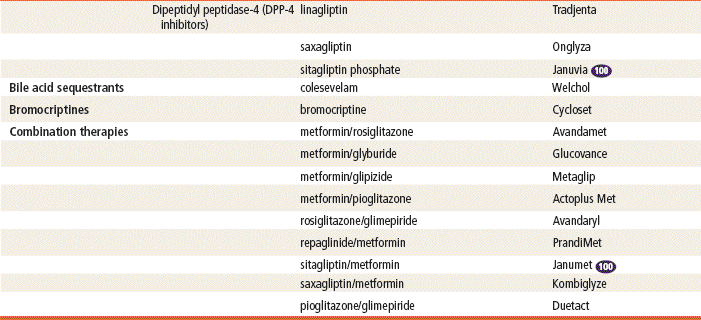
DPP-4 Inhibitors
Endogenous GLP-1 is rapidly inactivated by the enzyme dipeptidyl peptidase-4 (DPP-4). Therefore, drugs that inhibit DPP-4 will prolong the activity of GLP-1. The first DPP-4 inhibitor, sitagliptin, was approved by the FDA in October 2006 as a once-daily oral medication. It can be used as monotherapy or can be combined with metformin or a thiazolidinedione. It reduces fasting and postprandial hyperglycemia in patients with type 2 diabetes and does not cause weight gain or hypoglycemia. Hypersensitivity to this product is the only listed contraindication for sitagliptin. Saxagliptin and linagliptin are also available for use. Linagliptin’s advantage is that it does not need to be decreased for renal or hepatic dysfunction, and it appears to have less cardiovascular risk than the other DPP-4 inhibitors; however, this requires further study.
Bile Acid Sequestrants
Colesevelam, as an add-on medication for type 2 diabetes, reduces cholesterol and fasting blood glucose levels by binding bile acids and cholesterol for excretion. It is associated with only modest A1c lowering (0.5%). Although it is not considered a first-line agent for serious hyperglycemia, it can be used by patients who can benefit from the modest effects of its dual actions. A clear advantage is that it does not cause hypoglycemia or weight gain and is useful for people who cannot tolerate statins. Side effects are constipation, triglyceride elevation, and risk for interference with oral agent absorption.
Dopamine-2 Agonists
Bromocriptine mesylate is a dopamine receptor agonist typically used to treat Parkinson’s disease and recently approved for use with type 2 diabetes. It works by altering hypothalamic metabolism and increasing insulin sensitivity. This centrally acting antidiabetic agent will lower postprandial glucose levels, hepatic glucose production, triglyceride and free fatty acid levels, and A1c by 0.5% to 0.7%. Side effects include dizziness, nausea, fatigue, and rhinitis.
Treatment Principles
Generally accepted guidelines for managing diabetes have been established by the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (ACE). Although subtle differences are evident, most guidelines are similar. See http://care.diabetesjournals.org/content/35/Supplement_1/S11.full.pdf+html and http://care.diabetesjournals.org/content/35/3/660.1.full.
Cardinal Points of Treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Top 100 drug;
Top 100 drug;  key drug.
key drug.