Diabetes Mellitus
LEARNING OBJECTIVES
• INTRODUCTION
More than 25 million Americans have diabetes mellitus, 90% to 95% of whom have Type 2 diabetes mellitus. The economic burden of diabetes is almost $250 billion annually. Because most patients with Type 2 diabetes have the metabolic syndrome, with concomitant hypertension and hyperlipidemia, the average number of medications per patient ranges from 4.1 to 5.9. Because of the reliance on medication to control diabetes and its comorbid conditions, the pharmacist is a key member of the health care team in the management of patients with diabetes. Not surprisingly, there are numerous studies demonstrating the pharmacist’s effectiveness in managing Type 2 diabetes. Therefore, this chapter focuses on the competencies required by pharmacists to assume a primary care role in the treatment of patients with Type 2 diabetes mellitus.
• CLASSIFICATION/ETIOLOGY
There are two main forms of diabetes mellitus (Table 20.1). The first is Type 1 diabetes, also previously known as insulin-dependent diabetes mellitus (IDDM) or juvenile onset diabetes. Type 1 diabetes is an autoimmune disease that results in the destruction of the β cells in the islets of Langerhans in the pancreas. Once 80% to 90% of the β cells are destroyed, severe glucose intolerance develops because of the lack of insulin. With little or no insulin available, patients are unable to use glucose. In response, the body breaks down proteins and fats for energy, frequently resulting in the development of ketoacidosis, which can be fatal. Patients with Type 1 diabetes require insulin injections due to the lack of insulin production to control glucose and prevent ketoacidosis. The exact mechanism for this autoimmune process is unknown, but patients who develop Type 1 diabetes have a genetic predisposition and the β-cell destruction is triggered by either infectious, chemical, or dietary agents. Generally, patients who develop Type 1 diabetes mellitus do so as children or adolescents. In contrast to patients with Type 2 diabetes, who are overweight, patients with Type 1 are usually of normal body shape and weight. In some cases, the destruction is rapid, and the onset is sudden. In others the destruction is slower and may take up to a year to fully develop signs and symptoms.
| TABLE 20.1 | Classification of Diabetes Mellitus |
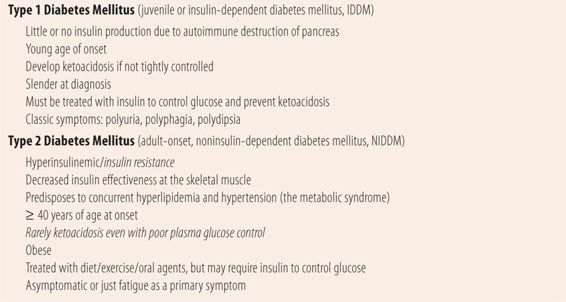
Type 2 diabetes, also previously known as adult onset or noninsulin dependent (NIDDM), usually occurs in patients over 40 years of age. Patients tend to be obese, and rather than having no insulin tend to be hyperinsulinemic early in the disease due to insulin resistance. This prevents insulin from entering cells to provide energy. Because muscles are not getting enough energy, they signal the liver to produce more glucose, causing glycogenolysis and gluconeogenesis. Insulin resistance also contributes to the development of hypertension and dyslipidemia. Therefore, most patients with Type 2 diabetes also have dyslipidemia and hypertension. Those three comorbid diseases along with other factors create the metabolic syndrome, which greatly increases the risk of cardiovascular disease (Table 20.2). Because there is some insulin effect, patients with Type 2 diabetes mellitus rarely develop ketoacidosis. Type 2 diabetes can be managed by diet and exercise alone, oral antidiabetic agents, or a combination of both. Eventually many patients will require insulin therapy to optimize control of their plasma glucose.
| TABLE 20.2 | The Metabolic Syndrome |

• DIAGNOSIS
The classical symptoms of diabetes include polyuria (excess/frequent urination), polydipsia (excessive thirst), and polyphagia (excessive hunger). Historically these occur in patients with Type 1 diabetes mellitus, but also can occur in patients with Type 2 diabetes. Polyuria is caused by high-plasma glucose levels exceeding the kidney’s threshold to reabsorb glucose. Once the plasma glucose exceeds 160 mg/dL, most patients spill glucose into the urine, creating a hyperosmolar state that causes water to be pulled into the tubules. Most patients notice it as nocturia (frequent urination at night), since they cannot sleep through the night without being awakened by the urge to urinate several times. The higher the plasma glucose levels, the greater the chance of developing dehydration. Excess plasma glucose also creates a hyperosmolar state. Both the polyuria-induced dehydration and the hyperosmolar state stimulate polydipsia (excessive thirst) in an attempt to correct those conditions. Finally, because glucose cannot be effectively utilized, the body is starved for energy so it stimulates polyphagia (excessive eating) in an attempt to correct the glucose/energy deficit.
Symptoms of Type 2 diabetes mellitus are not quite as obvious as in Type 1 diabetes mellitus because they occur more slowly. Fatigue is the most common symptom followed by nocturia. However, patient recognition of nocturia or polyuria may be masked by other conditions associated with aging that also cause more frequent urination, e.g., benign prostatic hypertrophy in males and postbirth trauma conditions such as cystocele or uterine prolapse in postmenopausal women. Frequently, the first sign of diabetes is an episode of vulvovaginal candidiasis (VVC), candidal balanitis, or a cut that easily becomes infected or does not heal normally. Excess glucose in the urine and vaginal fluid predisposes women to developing UTI’s or candidiasis and plasma glucose levels above 200 mg/dL impair neutrophil response to any bacterial infection. Therefore, patients over 40 years of age, with VVC or unexpected bacterial infection, should be questioned for symptoms of Type 2 diabetes mellitus and after the infection has been cleared, a fasting plasma glucose should be done. A fasting plasma glucose of >126 mg/dL, a random plasma glucose of ≥ 200 mg/dL, or a A1C (hemoglobin A1C, glycosylated hemoglobin) ≥ 6.5% are all diagnostic of diabetes mellitus. A1C is the percentage of hemoglobin that has glucose attached. Fasting plasma glucose levels of 100 to 125 mg/dL are considered prediabetes, a situation that needs to be addressed by lifestyle changes (weight loss, diet, and exercise). Prediabetes, which is common in patients with the metabolic syndrome, is a warning sign that if the patient continues their present lifestyle they will eventually develop Type 2 diabetes mellitus.
• COMPLICATIONS
There are multiple severe complications in patients with suboptimally controlled diabetes mellitus. Excess levels of plasma glucose directly and indirectly damage blood vessels and nerves, leading to many of the complications. Excess glucose effects on the immune system, coupled with neuropathy and microangiopathy can combine to cause the diabetic foot. The important point is that controlling plasma glucose levels prevents or delays the complications of diabetes. Many complications are caused wholly or in part by microangiopathy, the thickening of the basement membrane of tiny blood vessels (precapillary arterioles and capillaries) in all parts of the body. The thickening of blood vessel walls eventually leads to ischemia and cell death. Nephropathy, neuropathy, and retinopathy all have elements of microangiopathy in their etiology. Macroangiopathy refers to the ability of diabetes mellitus to accelerate the rate of atherosclerosis in larger blood vessels, leading to myocardial infarction, stroke, and peripheral artery disease.
In uncontrolled Type 1 diabetes mellitus, the obvious and potentially fatal complication is diabetic ketoacidosis. In patients with Type 2 diabetes, uncontrolled diabetes mellitus can occasionally lead to hyperosmolar non-ketotic coma. Typically plasma glucose levels are well in excess of 1000 mg/dL (but can occur with >600 mg/dL) and patients present with symptoms of dehydration (polyuria, polydipsia, fever, warm dry skin without sweating). Other symptoms include vision impairment, confusion, hallucinations, weakness, and somnolence. Because less than 20% of patients develop a coma, it is now called hyperosmolar, hyperglycemic, non-ketotic syndrome (HHNS). Typically, it occurs as the result of a combination of poor control and a bacterial infection, which further elevates plasma glucose.
Retinopathy
Diabetic retinopathy is the leading cause of blindness in the United States. Microangiopathy of tiny retinal blood vessels lead to hypoxia of retinal tissue and cellular death and formation of scar tissue. The vessels are also weaker and develop aneurysms, which may rupture, leading to small hemorrhages. The lack of oxygen eventually causes the proliferation of multiple small blood vessels to overcome the hypoxia. Macular edema also occurs. The combination of proliferation of small arterioles and macular edema eventually leads to visual loss. An annual dilated retinal examination by an eye professional is required to detect and monitor retinopathy. Findings of diabetic retinopathy during retinal examination include neovascularization, hard exudates (yellow dots), which are lipid residues of serious leakage from damaged capillaries, soft or cotton wool exudates, which are retinal nerve fiber microinfarcts, and hemorrhages of many shapes and sizes reflecting bleeding in various layers of the retina.
Nephropathy
Diabetes is the leading cause of end-stage renal disease (ESRD) in the United States. Excess blood glucose levels directly cause mesangial expansion, thickening of the basement membrane of the glomerulus, and eventually glomerulosclerosis. Since hypertension by itself causes nephropathy by a different mechanism, patients with Type 2 diabetes are far more likely to develop ESRD. As changes occur in the glomerulus, protein normally retained in the blood stream begins to leak out, causing proteinuria, the earliest sign of decreased renal function. Faced with high blood pressure and high blood glucose levels, renal function progressively decreases. Because of this process, an annual test for microalbuminuria and renal function are required.
Neuropathy
Diabetic neuropathy is the leading cause of nontraumatic amputations in the United States. While the exact cause of diabetic neuropathy is unknown, it appears to be multifactorial. Polyols, glucose by-products, accumulate in peripheral nerves and cause nerve damage. Other advanced glycation end products stimulate the immune system to attack nerves. High glucose levels cause nerves to produce reactive oxygen molecules that are thought to damage neuronal mitochondria. Finally, microangiopathy eventually causes nerve ischemia and death. The result of these processes is progressive loss of sensation usually starting in the feet. In addition to the loss of sensation, some patients will develop lancinating neuropathic pain, which can manifest itself on the bottom of the feet as burning sensations. Starting in the peripheral nerves, diabetic neuropathy can eventually progress to affect the autonomic nervous system where it manifests itself as impotence in males, vaginal dryness in women. Diabetic gastroparesis or paralysis of the gastrointestinal tract eventually develops. Gastroparesis usually occurs in patients who have had peripheral diabetic neuropathy for many years and manifests itself as a feeling of early fullness, belching and bloating, constipation, or diarrhea.
While the pain of diabetic neuropathy can be difficult to treat, the loss of sensation in the feet has far more dangerous consequences, the diabetic foot. When normal patients step on a stone, they sense the discomfort and shift their weight to prevent the stone from breaking the skin. Similarly, pain from tight fitting shoes alerts normal patients to make footwear changes or pad the tight area to prevent blister formation. Patients with diabetic neuropathy cannot feel the stone or the tightness and both may cause significant damage to the skin of the foot. In patients with diabetic neuropathy due to poorly controlled diabetes, microangiopathy to the small blood vessels in the skin is usually well advanced, leaving layers of skin and subdermal tissue with markedly reduced blood flow. The inoculation of bacteria from a stone breaking the skin or a blister rupturing creates a source of infection. Because of the decreased circulation, neutrophils have difficulty reaching the location. In addition, if the plasma glucose is >200 mg/dL, neutrophils respond poorly to chemotactic factors. Finally, because of poor circulation, it can be difficult to get enough antibiotic to the site of infection. Similarly, this microangiopathy can by itself cause ulcerations due to chronic ischemia and deeper ischemia, which eventually leads to gangrene and amputation (Figure 20.1). That is why a comprehensive diabetic foot examination is so important and is required at least annually. The diabetic foot examination checks for peripheral neuropathy with the monofilament examination and vibratory sensation (Table 20.3). Checking for the presence of the dorsalis pedis and posterior tibial pulses evaluates peripheral artery disease (macroangiopathy). Loss of hair on the toes, skin atrophy, delayed capillary fill time, and differences in temperature between feet or between parts of the same foot, all indicate microangiopathy. Care should be taken in evaluating capillary refill time especially in patients with advanced neuropathy. Use of the toe pad on the end of the big toes is the safest. Some references suggest squeezing the toes superiorly to inferiorly, with the thumb on the nail. This is not a good idea since hard pressure may cause a break in the skin from the compressed toenail, setting up a nidus for infection. Finally, looking for potential sources of trauma and infection involves looking for ingrown toenails, blisters, cuts, ulcers, and red areas caused by friction from footwear. Callouses bear special mention. In a patient with peripheral neuropathy, a callous (thick hard skin) is just like a stone. While not breaking the skin, the pressures can cause damage to subcutaneous tissue underneath the callous, which can eventually lead to gangrenous conditions and amputations. Patients are encouraged to inspect their own feet daily for cuts, sores, blisters, and ulcers. A metal camp mirror is very useful for this purpose. Once patients begin to lose sensation in their feet, all foot care needs to be done by a podiatrist. Self-care, even trimming toenails, is not wise. Some experts recommend a brief screening at each provider visit (every 3 to 6 months) since it takes only a few minutes and has been shown to reduce the incidence of amputations.
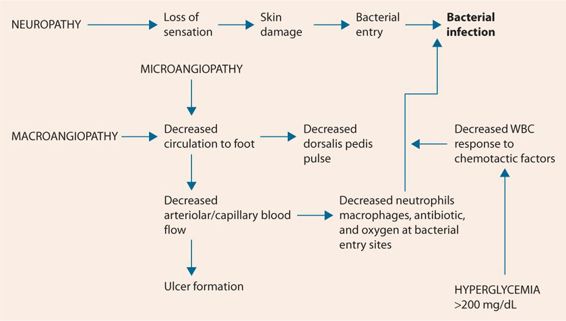
FIGURE 20.1 Pathophysiology of the diabetic foot.
| TABLE 20.3 | Diabetic Foot Examination |

Macroangiopathy
In addition to microangiopathy’s effect on arterioles and capillaries, poorly controlled diabetes accelerates the rate of atherosclerosis by reducing the effectiveness of protective enzymes, while enhancing inflammation among atherosclerotic plaques in larger blood vessels. These deleterious changes increase the incidence of myocardial infarctions, atherosclerotic stroke, and peripheral artery disease. Patients with Type 2 diabetes mellitus also have dyslipidemia and hypertension due to the insulin resistance in the metabolic syndrome, both of which also accelerate atherosclerosis. In establishing target LDL cholesterol levels, diabetes is considered the equivalent of a history of coronary artery disease. Macroangiopathy, through atherosclerosis in larger vessels, combines with microangiopathy to eventually worsen circulatory problems in the extremities.
Infection-Prone
Some textbooks categorize patients with diabetes as immunosuppressed or immunocompromised because they appear to be more susceptible to a wide variety of infections. In reality, under normal circumstances, patients with well-controlled diabetes have similar incidences of bacterial, fungal, and viral infections as do patients without diabetes. However, high blood glucose levels (uncontrolled diabetes mellitus) do increase the risk for fungal and bacterial infections. First excess glucose in urine, sweat, and vaginal fluids predispose patients to urinary tract infections, vulvovaginal candidiasis, candidal balanitis, and soft tissue infections, because the glucose is fuel for microbial growth and has other direct effects to enhance microbial growth. In addition, at plasma glucose levels >200 mg/dL neutrophils and macrophages do not respond as well to chemotactic factors released at the site of bacterial infections, reducing the body’s ability to fight infection. However, even patients who normally have excellent control of their blood glucose will be at increased risk during periods surrounding surgery or other trauma due to the release of substances, such as cortisol, that increase blood glucose levels as part of the physiological process to speed the repair of damaged tissue. Similarly, when bacterial infections do occur in patients with excellent control, plasma glucose levels tend to rise because of similar physiological defense and repair processes. In patients who present with a bacterial infection and a mildly elevated plasma glucose level, a fasting plasma glucose should be done several weeks after the infection has cleared to determine if the patient has diabetes or prediabetes.
• INITIAL VISIT
An initial visit for a patient with diabetes has two major components. The first component is to confirm the diagnosis of diabetes mellitus and the metabolic syndrome, the presence of comorbid illness, e.g., dyslipidemia and hypertension, and the absence of secondary causes of elevated plasma glucose such as hypothyroidism. The second component is to assess for the presence and severity of complications due to diabetes, hypertension, and dyslipidemia. Initial evaluation will require a complete history and physical examination, with special emphasis on cardiovascular risk factors, peripheral neuropathy, vascular disease, and other potential causes for nocturia such as uterine prolapse or cystocele in females and benign prostatic hypertrophy in males. Because diet and exercise are important components of treatment of Type 2 diabetes, a thorough history of current dietary and exercise habits is an important part of the initial workup. Similarly, a smoking history is needed since smoking accelerates microangiopathy, atherosclerosis (macroangiopathy), and neuropathic complications of diabetes (Table 20.4).
| TABLE 20.4 | Initial Patient Visit Checklist |

• FOLLOW-UP VISITS
Follow-up visits cover evaluation of control of diabetes mellitus, compliance with the therapeutic regimen, and complications from the disease and the therapeutic regimen (Table 20.5). The general approach to a follow-up visit is to begin with broad open-ended questions to start the dialog about symptoms for each section (control, compliance, complications). Positive responses are followed up with more focused open-ended questions. Similarly, if specific information is not covered in response to broad questions, a focused open-ended question approach is used. Finally, a series of closed-ended questions can be used to double check for the presence of symptoms that may indicate poor control or complications due to the disease or medication regimen.
| TABLE 20.5 | Follow-Up Visit Checklist |


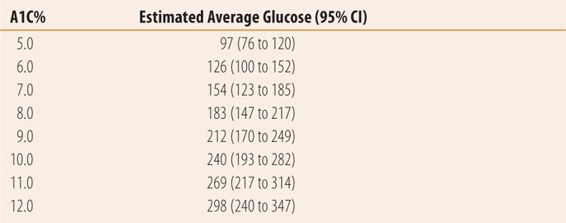
The A1C is the primary test used to evaluate disease control because it is a measure of average blood glucose over a 3-month period (Table 20.6). In interpreting the A1C, the last month represents half of the value and months 1 and 2 the other half. The target A1C is less than 7%. However, like with hypertension and dyslipidemia there is little difference between an A1C of 6.9 and 7.2 and pharmacists should be slow to add medications for small changes in A1C. Recent studies show that aggressive target values for glucose control, hypertension, and hyperlipidemia do not further reduce morbidity and mortality and in some cases negatively affect overall outcomes. The vast majority of benefit in preventing complications occurs at A1C levels below 8.0% so while achieving target A1C is important, it does not require heroic effort or instant change. Fatigue and nocturia are common symptoms of hyperglycemia that should lessen as A1C levels near target. Urinary tract infections, vulvovaginal candidiasis, candidal balanitis, and candidal rashes may also be symptoms of excess blood glucose. Some experts recommend that in addition to an A1C <7%, fasting or preprandial plasma glucose levels of 70 to 130 mg/dL should be an additional goal. Other experts, based on evidence that high postprandial glucose levels are the most damaging, suggest using 2-hour postprandial plasma glucose levels of less than 140 mg/dL as an additional goal.
| TABLE 20.6 | Relationship Between A1C and Estimated Average Glucose Levels |
While objective monitoring parameters for blood glucose control are well established, and therapy with medication, diet, and exercise consistently lower blood glucose levels, the same cannot be said of weight. Patients with Type 2 diabetes mellitus, who are treated with insulin or sulfonylureas, probably will all gain weight, even though the control of their diabetes improves!! While the exact mechanism is unknown, it can be problematic in that it is thought to potentially increase cardiovascular disease risk. More importantly, it is very frustrating to patients who have been told that the key to diabetes control is to lose weight. This patient frustration can negatively impact regimen adherence and eventually disease control. Metformin, by an unknown mechanism, largely prevents this therapy-induced weight gain, which averages 5 to 10 lb. In general, the higher the A1C, and the more aggressive the blood glucose lowering, the larger the weight gain. Part of the rationale in the new guidelines for using metformin as the initial agent in all patients with Type 2 diabetes mellitus is that if sulfonylureas or insulin must be added for better disease control at a later date, the metformin will prevent or lessen that weight gain. In addition, lowering plasma glucose levels more gradually seems to lessen the weight gain.
While self blood glucose monitoring (SBGM) has been the subject of much discussion in patients with Type 2 diabetes, there are some definite benefits for patients, particularly early in therapy. Two-hour postprandial readings along with a food diary can be used to help educate patients regarding the impact of types and portions of various foods on blood glucose levels. Some recommend the SBGM plus food diary along with nutrition consultation as the only activity for the first 2 weeks in newly diagnosed asymptomatic patients to reinforce the effects of diet on diabetes control. The effect of exercise on blood glucose can be determined by having the patient test blood glucose immediately after exercise and one hour-later. This is particularly important in patients on medications that may cause hypoglycemia. Some patients may choose a basal-bolus insulin regimen, where bolus doses are calculated based on carbohydrate intake and requires testing several times a day. Finally, SBGM has been shown to improve adherence with the therapeutic regimen. Once daily SBGM, with varying times of fasting, preprandial and 2-hour postprandial testing each day, can give the patient and the provider information that may assist in a more accurate adjustment of diet, exercise, and medication, while minimizing the testing burden.
While many of the commonly used medications today rarely cause hypoglycemia, all patients should be educated in the recognition of signs and symptoms of hypoglycemia and its treatment. Symptoms of hypoglycemia include those caused primarily by the body’s release of catecholamines in an attempt to raise blood glucose by glycogenolysis. Catecholamines and low blood glucose levels combine to cause behavioral changes ranging from light headedness or agitation to semiconscious or unconscious states (Table 20.7).
| TABLE 20.7 | Signs and Symptoms of Hypoglycemia |

In addition to evaluating the control of the patient’s diabetes, adherence to all aspects of the therapeutic regimen is evaluated, including diet, exercise, and medications. Finally, each follow-up visit should evaluate the presence and progression of complications due to the disease and drug therapy (Table 20.5). All patients require ophthalmoscopic examination, diabetic foot examination, and microalbuminuria testing annually. A brief diabetic foot examination should be done at each provider visit. In between annual examinations, responses to questions at follow-up visits about vision, neuropathy, and other complications will determine the need for more frequent evaluations. Once the first signs of neuropathy, nephropathy, and/or retinopathy are detected, evaluation and follow-up by the appropriate specialist is recommended, in addition to follow-up visits for routine care of diabetes mellitus. Similarly, routine dental care is important since high blood glucose levels increase the incidence of periodontal disease. Finally, most patients with Type 2 diabetes mellitus also have the metabolic syndrome and comorbid diseases of dyslipidemia and hypertension. Many of the questions and tests listed in Table 20.5 for complications of diabetes also apply to hypertension and dyslipidemia. For specific subjective and objective disease and medication monitoring parameters for comorbid disease, see Chapters 18 and 19.
• KEY REFERENCES
1. Anon. Standards of medical care in diabetes-2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
2. Anon. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(suppl 1):S81-S90.
3. Haas L, Marynuik M, Beck J, et al. National standards for diabetes self-management education and support. Diabetes Care. 2014;37(suppl 1):S144-S153.
4. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment. Diabetes Care. 2008;31:1679-1685.
5. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a work group of the American Diabetes Association and Endocrine Society. J Clin Endocrinol Metab. 2013;98:1845-1859.
6. Blevins T. Control of postprandial glucose levels with insulin in type2 diabetes. Postgrad Med. 2011;123:135-147.
7. Russell-Jones D, Kahn R. Insulin associated weight gain in diabetes, causes effects and coping strategies. Diabetes Obes Metab. 2007;9:799-812.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



