11 Diabetes mellitus
Diabetes mellitus is a clinical syndrome characterised by hyperglycaemia due to absolute or relative deficiency of insulin. Long-standing metabolic derangement can lead to the development of complications of diabetes, which characteristically affect the eye, kidney and nervous system. Diabetes occurs world-wide and its incidence is rising; 171 million people had diabetes in 2000, and this is expected to double by 2030. Diabetes is a major burden upon health-care facilities in all countries.
FUNCTIONAL ANATOMY, PHYSIOLOGY AND INVESTIGATIONS
NORMAL GLUCOSE AND FAT METABOLISM
After meals, blood insulin levels rise in response to a rise in blood glucose. Insulin is an anabolic hormone and is secreted from pancreatic β cells into the portal circulation. Insulin lowers blood glucose by suppressing hepatic glucose production and stimulating glucose uptake in skeletal muscle and fat.
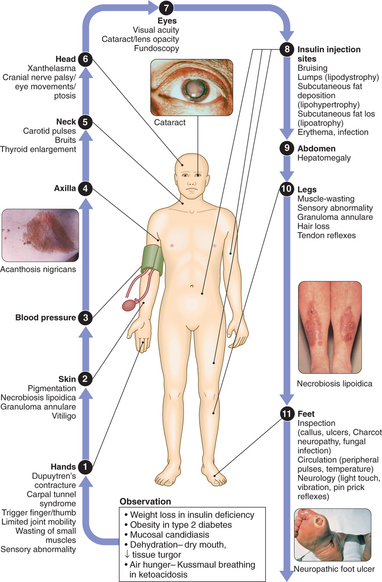
CLINICAL EXAMINATION OF THE PATIENT WITH DIABETES
Insulin stimulates lipogenesis and inhibits lipolysis. Lipolysis is stimulated by catecholamines and liberates FFAs, which can be oxidised by many tissues. Their partial oxidation in the liver produces ketone bodies, which can be oxidised and utilised as metabolic fuel. However, the rate of utilisation of ketone bodies by peripheral tissues is limited, and when production by the liver exceeds removal, hyperketonaemia results. Ketogenesis is enhanced by insulin deficiency and release of the counter-regulatory hormones that stimulate lipolysis.
AETIOLOGY AND PATHOGENESIS OF DIABETES
TYPE 1 DIABETES
Type 1 diabetes is a slowly progressive T cell-mediated autoimmune disease leading to destruction of the insulin-secreting β cells. Classical symptoms of diabetes occur only when 70–90% of β cells have been destroyed. Pathology shows insulitis (infiltration of the islets with mononuclear cells), in which β cells are destroyed, but cells secreting glucagon and other hormones remain intact. Islet cell antibodies can be detected before clinical diabetes develops and disappear with increasing duration of diabetes; however, they are not suitable for screening or diagnostic purposes. Glutamic acid decarboxylase (GAD) antibodies may have a role in identifying late-onset type 1 autoimmune diabetes in adults (LADA). Type 1 diabetes is associated with other autoimmune disorders, including thyroid disease (p. 342), coeliac disease (p. 446), Addison’s disease (p. 366), pernicious anaemia (p. 539) and vitiligo (p. 709).
Environmental factors
Reduced exposure to microorganisms in early childhood may limit maturation of the immune system and increase susceptibility to autoimmune disease (the ‘hygiene hypothesis’). Viral infections including mumps, Coxsackie B4, retroviruses, congenital rubella, cytomegalovirus and Epstein–Barr virus might cause some forms of type 1 diabetes. Bovine serum albumin (BSA—a constituent of cow’s milk) has been implicated in triggering type 1 diabetes, since infants who are given cow’s milk are more likely to develop type 1 diabetes in later life than those who are breastfed. Stress may precipitate type 1 diabetes by stimulating the secretion of counter-regulatory hormones and possibly by modulating immune activity.
Metabolic disturbances in type 1 diabetes
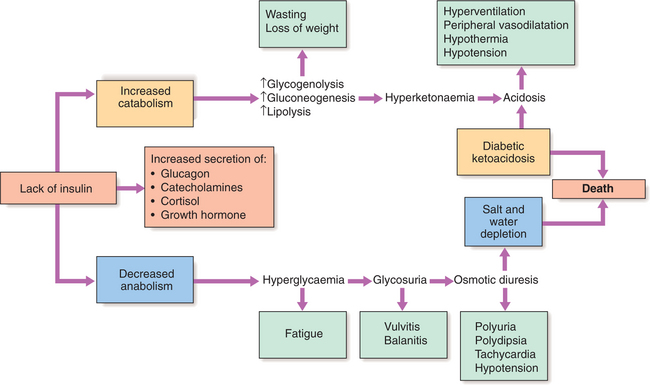
Patients with type 1 diabetes present when adequate insulin secretion can no longer be sustained. High glucose levels may be toxic to the remaining β cells so that profound insulin deficiency rapidly ensues. Insulin deficiency is associated with the metabolic sequelae shown in Figure 11.1. Hyperglycaemia leads to glycosuria and dehydration, which in turn induces secondary hyperaldosteronism. Unrestrained lipolysis and proteolysis result in weight loss, increased gluconeogenesis and ketogenesis. When generation of ketone bodies exceeds their metabolism, ketoacidosis results. Secondary hyperaldosteronism encourages urinary loss of K+. Thus patients usually present with a short history of hyperglycaemic symptoms (thirst, polyuria, fatigue and infections) and weight loss, and may have developed ketoacidosis.
TYPE 2 DIABETES
Insulin resistance: Excessive production of glucose in the liver and under-utilisation of glucose in skeletal muscle result from resistance to the action of insulin. Type 2 diabetes is often associated with other medical disorders, which when they coexist are termed ‘metabolic syndrome’ (syndrome X, Box 11.1), with a predisposition to insulin resistance being the primary defect. It is strongly associated with macrovascular disease (coronary, cerebral, peripheral) and an excess mortality.
Metabolic disturbances in type 2 diabetes
In some patients, presentation is late and pancreatic β-cell function has declined to the point where there is profound insulin deficiency. These patients may present with weight loss, although ketoacidosis remains rare. Intercurrent illness, e.g. with infections, increases the production of counter-regulatory hormones (cortisol, growth hormone, catecholamines), resulting in more severe hyperglycaemia and dehydration (see hyperosmolar non-ketotic coma, p. 395). Dyslipidaemias are also common in patients with type 2 diabetes.
PRESENTING PROBLEMS
NEWLY DISCOVERED HYPERGLYCAEMIA
Establishing the diagnosis of diabetes
In a symptomatic patient, the diagnosis may be confirmed by a random plasma glucose concentration ≥11.1 mmol/l (200 mg/dl) or a fasting plasma glucose concentration ≥7 mmol/l (126 mg/dl). In an asymptomatic patient two samples are required. An oral glucose tolerance test (OGTT) is indicated when plasma glucose levels are elevated but not diagnostic of diabetes: fasting range 6.1–7.0 mmol/l (110–126 mg/dl), or random plasma glucose range 7.8–11.0 mmol/l (140–199 mg/dl). The WHO criteria for diagnosing diabetes mellitus are shown in Box 11.2 and are based on the risk of developing microvascular disease. Patients who do not meet the criteria for diabetes may have ‘impaired glucose tolerance’ (IGT) or ‘impaired fasting glucose’ (fasting glucose 6.1–6.9 mmol/l (110–125 mg/dl)). These patients have an increased risk of progression to frank diabetes and of macrovascular disease.
11.2 ORAL GLUCOSE TOLERANCE TEST: WHO DIAGNOSTIC CRITERIA ![]()
| Glucose concentrations | ||
|---|---|---|
| Venous plasma mmol/l (mg/dl) | Capillary whole blood mmol/l (mg/dl) | |
| Diabetes | ||
| Fasting | ≥7.0 (≥126) | ≥6.1 (≥110) |
| 2 hrs after glucose load | ≥11.1 (≥200) | ≥11.1 (≥200) |
| Impaired glucose tolerance | ||
| Fasting | <7.0 (<126) | <6.1 (<110) |
| 2 hrs after glucose load | 7.8–11.0 (140–199) | 7.8–11.0 (140–199) |
When diabetes is confirmed, other investigations should include:
Clinical assessment
The clinical features of the two main types of diabetes are compared in Box 11.3. Overlap occurs particularly in age at onset, duration of symptoms and family history. Typical type 2 diabetes increasingly occurs in obese young people. Older adults may have evidence of autoimmune activity against β cells, a slowly evolving variant of type 1 diabetes (LADA). More than 70% of patients with type 2 diabetes are overweight, 50% have hypertension and hyperlipidaemia is common.
11.3 COMPARATIVE CLINICAL FEATURES OF TYPE 1 AND TYPE 2 DIABETES ![]()
| Type 1 | Type 2 | |
|---|---|---|
| Typical age at onset | <40 yrs | >50 yrs |
| Duration of symptoms | Weeks | Months to years |
| Body weight | Normal or low | Obese |
| Ketonuria | Yes | No |
| Rapid death without treatment with insulin | Yes | No |
| Autoantibodies | Yes | No |
| Diabetic complications at diagnosis | No | 25% |
| Family history of diabetes | Uncommon | Common |
| Other autoimmune disease | Common | Uncommon |
Management
LONG-TERM SUPERVISION OF DIABETES
Diabetes is a complex disorder, which progresses in severity with time. Patients with diabetes should therefore be seen at regular intervals for life. A checklist for follow-up visits is given in Box 11.4. The frequency of visits varies from weekly during pregnancy to annually in well-controlled type 2 diabetes.
11.4 CHECKLIST FOR FOLLOW-UP OF PATIENTS WITH DIABETES MELLITUS ![]()
| Body weight (BMI) | |
| Urinalysis (fasting) | Glucose, ketones, macro- and microalbuminuria |
| Glycaemic control | HbA1c, inspection of home blood glucose monitoring record |
| Hypoglycaemic episodes | Number and time of day of severe and mild episodes, nature of symptoms, awareness |
| BP | |
| Eye examination | Visual acuity, ophthalmoscopy, digital photography |
| Lower limbs | Peripheral pulses, tendon reflexes, sensory: proprioception, vibration, light touch |
| Feet | Callus skin indicating pressure areas, nails, podiatry, ulceration, deformity |
DIABETIC KETOACIDOSIS (DKA)
Ketoacidosis is a major medical emergency, principally occurring in people with type 1 diabetes. A significant number of newly diagnosed diabetic patients present in ketoacidosis. In established diabetes, any form of stress, particularly infection, can precipitate DKA. Patients lose their appetite, and stop or reduce their dose of insulin in the mistaken belief that less insulin is required. No obvious precipitating cause can be found in many cases. The average mortality in developed countries is 5–10% and is higher in the elderly. The cardinal biochemical features of DKA are:
Hyperglycaemia causes an osmotic diuresis leading to dehydration and electrolyte loss. Ketosis is caused by insulin deficiency, exacerbated by stress hormones (e.g. catecholamines) resulting in unrestrained lipolysis supplying FFAs for hepatic ketogenesis. When this exceeds the capacity to metabolise acidic ketones, these accumulate in blood. The resulting acidosis forces hydrogen ions into cells, displacing potassium ions, which are lost in urine or through vomiting. The average loss of fluid and electrolytes in moderately severe DKA in an adult is shown in Box 11.5. Patients with DKA have a total body potassium deficit but this is not reflected by plasma potassium levels, which may initially be raised due to disproportionate water loss. However, once insulin is started, plasma potassium can fall precipitously due to dilution by i.v. fluids, potassium movement into cells, and continuing renal loss of potassium.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree