http://evolve.elsevier.com/Edmunds/NP/
This chapter discusses preparations used for skin, nail, and hair problems. These preparations are used for an extremely large array of dermatologic problems. See each section for more specific indications. General issues in topical medications are discussed; then corticosteroids, antiinfectives, acne medications, and local anesthesia are discussed, each in a separate section. The purpose of this chapter is to discuss the most common of the various available agents. It does not attempt to address all of the preparations or the myriad indications for these agents. This chapter focuses on the topical use of these products. See appropriate chapters for more information about drugs from specific drug categories.
Therapeutic Overview of Dermatologic Agents
The primary function of the skin is as a barrier. It functions to protect and thermoregulate. The skin is involved in the immune response, biochemical synthesis, and sensory detection. The skin’s barrier function is compromised when the skin has been damaged or when inflammation is present.
The skin, the largest organ of the body, consists of three distinct layers: epidermis, dermis, and subcutaneous tissue (Figure 13-1). The epidermis is the outer layer of the skin. The thickness of the epidermis ranges from 0.05 mm on the eyelids to 1.5 mm on the palms and soles. Five layers make up the epidermis. Basal cells form a single layer of cells that make up the innermost layer of the epidermis. These basal cells divide to form keratinocytes. The other layers are formed as keratinocytes change until they migrate to the outer layer to become the major component of the stratum corneum. The stratum corneum provides protection to the skin by acting as a barrier. The thicker the epidermis, the greater the barrier protection.
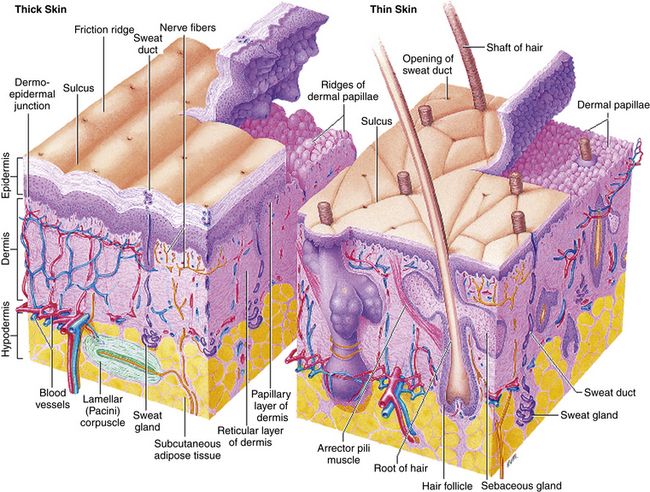
FIGURE 13-1 Structure of the skin. From Thibodeau GA, Patton KT: Anatomy and physiology, ed 7, St Louis, 2010, Mosby
The dermis, similar to the epidermis, varies in thickness, ranging from 0.3 mm on the eyelid to 3 mm on the back. Three types of connective tissue—collagen, elastic tissue, and reticular fibers—make up the dermis. Different from the epidermis, the dermis is made up of nerves, blood vessels, hair follicles, and apocrine and eccrine glands.
Subcutaneous tissue is the deepest layer. Distribution is dependent on sex characteristics. Age, heredity, and caloric intake also influence distribution. The subcutaneous tissue provides padding and insulation to the underlying structures.
New research has suggested that tattoo inks may include carcinogens (such as benzopyrene) and hormone disruptors (such as dibutyl phthalate, which can mimic estrogen or disrupt testosterone), and their injections into skin with small needles are linked to allergic rashes, chronic skin reactions, infection, and inflammation from sun exposure. The Food and Drug Administration has launched new studies to investigate the long-term safety of the inks, including what happens when they break down in the body or interact with light. It appears that when skin cells containing ink are killed by sunlight or laser light, the ink breakdown products could spread throughout the body. Research already has shown that tattoo inks migrate into people’s lymph nodes, but the connection of tattoo inks to malignancies such as melanoma, basal cell carcinomas, squamous cell carcinomas, and keratoacanthomas is not clear. The long-term health risks posed by tattoo inks are unknown. There may be potential effects on fetuses and infants—for example, in infant boys, feminization of the reproductive tract may occur with phthalate exposure.
Pathophysiology
Three types of primary skin lesions have been identified: the macule, the papule, and the vesicle. Variations of these lesions are named according to the size of the lesion. Secondary lesions include erosion, ulcer, fissure, crust, lichenification, atrophy, excoriation, scar, and keloid.
When one is describing a skin lesion, it is important to accurately name the type of lesion by using this terminology. Location on the body and pattern of distribution are important. Timing and course should include whether the onset was acute or insidious. Also, the presence of any systemic signs and symptoms should be noted.
Mechanism of Action
Topicals work by being absorbed into the skin. Their effect is local. The specific mechanisms of action are discussed in each separate section. Topical preparations are available in a bewildering array of products, including many combination products, corticosteroid products of different strengths, and antiinfectives of every kind. The primary care provider should become familiar with a few products and treatment measures rather than trying to master the complete array. Superficial skin infections and acne are commonly treated in primary care. If the patient does not respond to standard care, he is referred to a dermatologist. Primary care providers should be able to identify suspicious lesions and refer patients promptly to dermatology; such patients frequently require surgical treatment.
Where applicable, specific information regarding standard guidelines, evidence-based recommendations, cardinal points of treatment, pharmacologic treatment, and nonpharmacologic treatment is provided later in the chapter.
Topical therapy is unique because the skin is directly accessible for both diagnosis and therapy. Drugs used to treat skin problems can be applied directly to the site. All topical agents can also be absorbed systemically. Consider the adverse effects of the systemic medication when you are ordering topical agents.
Factors that affect the extent of drug absorption into the skin include the status of the skin, the characteristics of the drug, and the characteristics of the administration vehicle. Absorption is increased when the skin is broken or inflamed. In addition, absorption increases in cases in which skin integrity is compromised or the skin is thinner. Because of the vascular composition of the skin, mucous membranes absorb medication in high concentrations. The vehicle or base affects percutaneous absorption (Table 13-1). The vehicle may hydrate the outer layer of skin by preventing water loss. With improved hydration, the absorption of medication and the depth of penetration are enhanced.
Absorption of topical medications is slow and incomplete compared with drugs given orally. For optimal absorption, apply them to moist skin either immediately after bathing or after wet soaks.
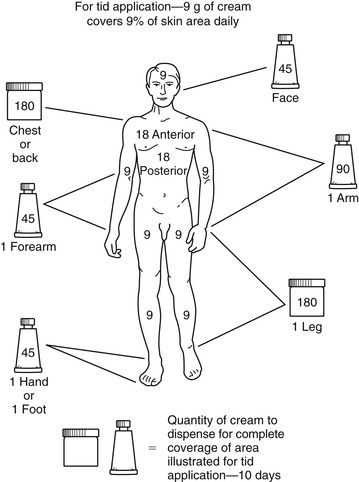
Prescribing the appropriate amount of topical medication is important. Too large a tube may be very costly to the patient, yet a small tube may not include enough medication to cover the entire area. To estimate the amount that should be prescribed, the rule of nines can be used (Figure 13-2).
Treatment Principles
Creams, Ointments, and Pastes
Lotions
Patient Variables
Patient Education
Topical medications come in many forms. Each has an effective and appropriate method of application. Topical medications should be applied thinly or lightly. The application of extra amounts usually does not provide increased benefit. Teach the patient the correct method of application for the particular preparation prescribed.
Topical Corticosteroids
For a listing, see Table 13-2.
TABLE 13-2
Topical Corticosteroids Ranked by Potency∗
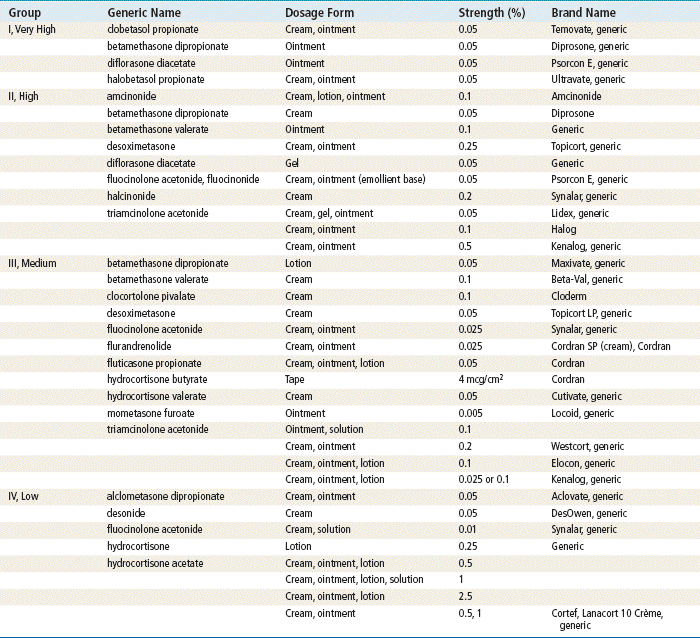
∗Range: group I (very potent) to group IV (least potent).
Therapeutic Overview of Topical Corticosteroids
Toxicity to topical corticosteroids, although not common, is the same as the toxicity that occurs when corticosteroids are given systemically. Other common adverse effects include atrophy, striae, telangiectasia, purpura, acne, perioral dermatitis, and steroid rosacea. Glaucoma and cataracts may develop after prolonged use of corticosteroids around the eyes. The risk of side effects is increased when topical corticosteroids are used over a prolonged time, under occlusion, on the face, and on intertriginous locations.
Mechanism of Action
Topical corticosteroids diffuse across cell membranes and induce cutaneous vasoconstriction commensurate with their potency. This results in a local antiinflammatory effect. The topical route is elected when a local effect is preferred to the systemic effect produced by the same product given orally. Topical corticosteroids inhibit the migration of macrophages and leukocytes into the area by reversing vascular dilation and permeability of small vessels in the upper dermis. See Chapter 51 for further information.
Treatment Principles
No general standardized guidelines are found. There are some guidelines for treatment of specific diseases or skin conditions. See www.guidelines.gov for specific conditions. (See Table 13-4.)
Cardinal Points of Treatment
Pharmacologic Treatment
Topical corticosteroids are ranked according to potency, with group I as the most potent and group VII as the least potent. It is usually sufficient to divide them into high-, medium-, and low-potency groups. Frequently, weaker strengths are used because they are considered to be safer. However, adequate strength is necessary for a therapeutic response. Weak OTC hydrocortisone is not effective against many dermatoses; a medium-strength steroid from class III or IV is often more effective. If the patient does not respond, treatment should be reevaluated.
Potency is the most important variable when a topical steroid corticosteroid is selected. A drug from each potency level should be chosen to meet the prescriptive needs of the patient. The potency of a steroid is not determined by its strength but by vasoconstrictor assays.
Vasoconstrictor assays measure skin blanching when an agent is applied to skin under occlusion. A difference in potency may be noted between generic and name brand corticosteroid equivalents. Pharmacists are allowed to substitute generic drugs unless the health care provider requests “No substitutions” or “Brand necessary.”
Use low-potency agents in children, on large areas, for mild conditions, and on body sites that are especially prone to corticosteroid damage, such as the face, scrotum, axilla, flexures, and skin folds.
Reserve high-potency agents for areas and conditions that are resistant to treatment with milder agents; these may be alternated with milder agents. Short-term intermittent therapy with the use of high-potency agents may be more effective and may cause fewer adverse effects than continuous treatment with low-potency agents. No fluorinated or high-potency corticosteroid should be used on the face.
For all groups, apply the product sparingly two to four times a day. Adequate results are usually achieved with twice-a-day application and a course of therapy of 2 to 6 weeks. To prevent rebound, do not discontinue treatment abruptly after long-term use of a potent agent. Instead, reduce the frequency of application, or use a lower-potency agent.
When desired results are not achieved, stop therapy for 4 to 7 days, and then resume treatment with a different agent. A more potent corticosteroid may be needed.
A drug from each potency level should be chosen to meet the prescriptive needs of the provider. Triamcinolone is commonly used because it is available generically and comes in a variety of strengths. Triamcinolone 0.5% is a good product of medium strength that is effective for many rashes seen in the office setting.
Group I
Topical corticosteroids in group I are super-potent agents. The amount of drug applied to the body per day and the duration of treatment must be carefully monitored. Per week, a maximum of 50 g of cream or ointment should be used. The duration of daily use of super-potent topical corticosteroids should not exceed 2 weeks, if possible. A period of 1 week is needed before a group I topical corticosteroid can be used again. This is called cyclic or pulse dosing. Diflorasone diacetate can be used under occlusion; betamethasone dipropionate cannot. Occlusive dressings should be used for no longer than 12 hours at a time. Renal suppression, skin atrophy, and other side effects are possible. Patients who use group I topical corticosteroids should be monitored closely for HPA suppression. Prescriptions should limit refills.
Group II
These are also considered high-potency corticosteroids. The risk of adverse effects is reduced if these agents are used for less than 6 to 8 weeks, except on the face or areas where opposing skin surfaces touch (e.g., skin folds of the groin, axillae, and breasts).
Groups III Through V
These are considered preparations of medium potency. They are the most commonly indicated preparations for skin conditions for which prescription strength corticosteroids are required. They often have different chemical bases. Treatment duration should not exceed 8 weeks (see previous section on group II).
How to Monitor
Patient Variables
Pediatrics
Pregnancy and Lactation
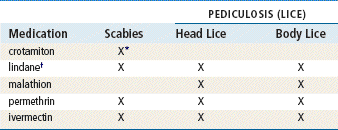
Antiinfectives
Mupirocin is the only drug in this class that is used exclusively for topical infection, and it is the only one discussed here in detail. Mupirocin represents a major advance because it allows topical treatment of impetigo. Bacitracin is very effective for most topical infections. Triple antibiotic ointments vary in their composition; usually, they contain neomycin, which causes a greater number of allergic reactions than are produced by other topical agents. See Chapter 58 for information on other drugs in this class.
Therapeutic Overview of Topical Antiinfectives
Most pathogens cultured from infected dermatoses are group A β-hemolytic streptococci, Staphylococcus aureus, Streptococcus pyogenes, or any of these pathogens in combination. Gram-negative infections of the skin are relatively rare.
Mechanism of Action
Mupirocin blocks the protein synthesis of bacteria by binding with the transfer ribonucleic acid (tRNA) synthetase.
Treatment Principles
How to Monitor
Specific Drugs
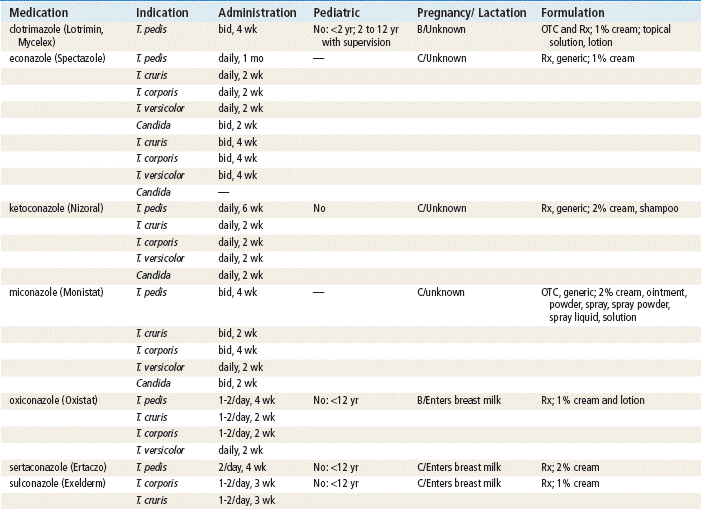
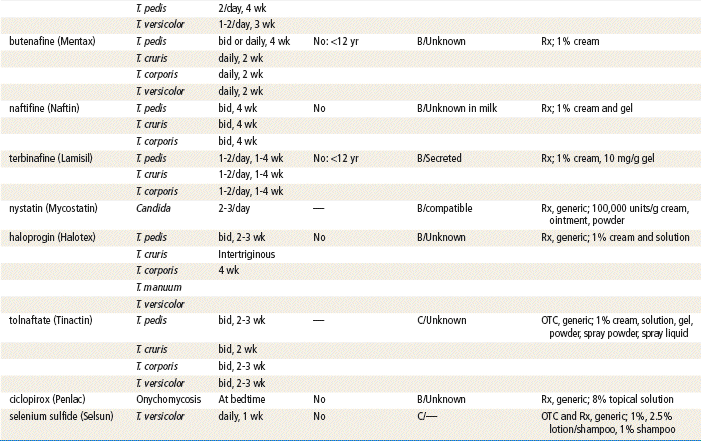
See Table 13-3 for dosage and administration recommendations for selected topical antibiotics.

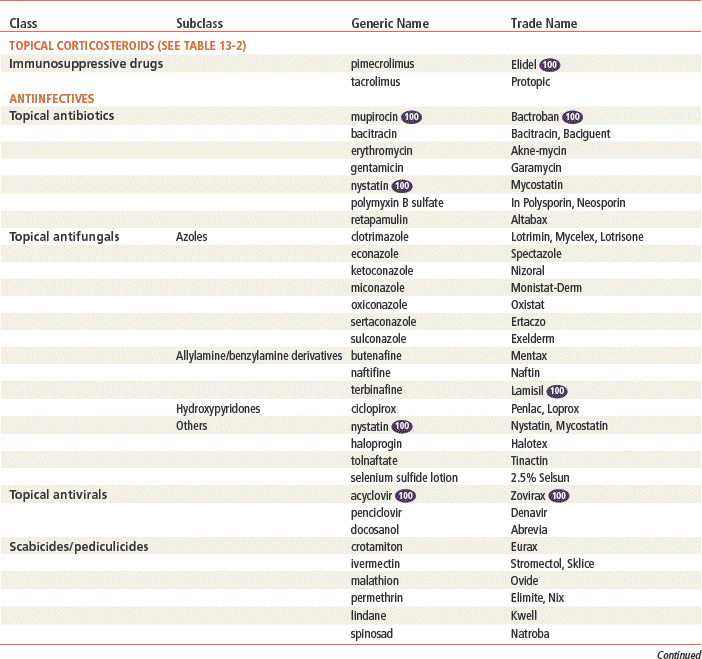
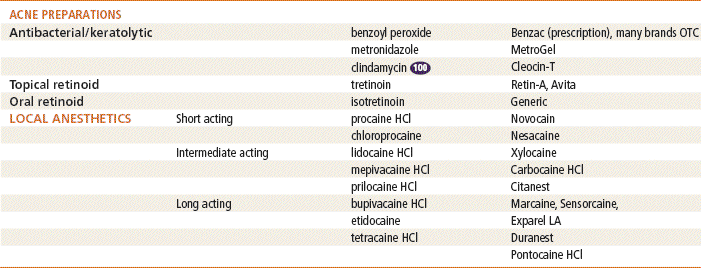
 Top 100 drug.
Top 100 drug.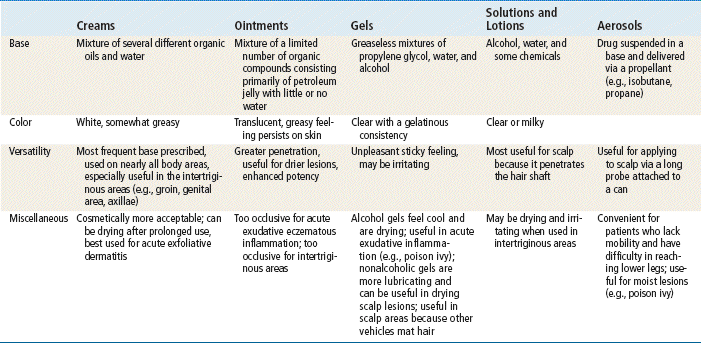
 The most common complication is suppression of the hypothalamic-pituitary-adrenal (HPA) axis, which usually is not associated with symptoms until the corticosteroid is stopped.
The most common complication is suppression of the hypothalamic-pituitary-adrenal (HPA) axis, which usually is not associated with symptoms until the corticosteroid is stopped. The dose of one corticosteroid does not correlate with the dose of another—that is, diflorasone diacetate (Psorcon) ointment 0.05% is much stronger than hydrocortisone 2.5%.
The dose of one corticosteroid does not correlate with the dose of another—that is, diflorasone diacetate (Psorcon) ointment 0.05% is much stronger than hydrocortisone 2.5%. Steroids that are potent are corticosteroids used for severe conditions or on a small area of the body.
Steroids that are potent are corticosteroids used for severe conditions or on a small area of the body. HPA axis suppression has occurred. Limit the amount, potency, and length of treatment.
HPA axis suppression has occurred. Limit the amount, potency, and length of treatment. Pimecrolimus permeates skin at a lower rate than tacrolimus.
Pimecrolimus permeates skin at a lower rate than tacrolimus.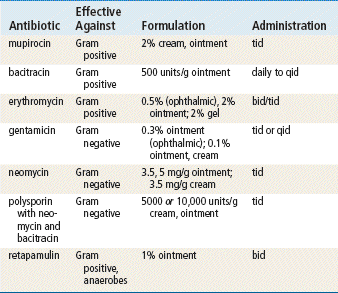
 Apply with caution to skin with impaired integrity because this may allow increased systemic absorption of the drug. Combination antibiotic and corticosteroid preparations often are not indicated because topical corticosteroids can impair the body’s ability to fight infection.
Apply with caution to skin with impaired integrity because this may allow increased systemic absorption of the drug. Combination antibiotic and corticosteroid preparations often are not indicated because topical corticosteroids can impair the body’s ability to fight infection.

