Delivery of biopharmaceuticals
Ijeoma F. Uchegbu and Andreas G. Schätzlein
Chapter contents
Key points
Introduction
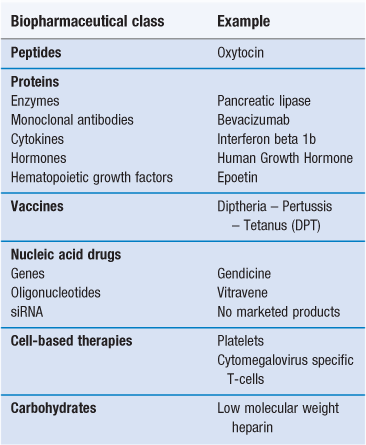
Biopharmaceuticals, also known as biologicals or biologics, are medicines in which the active is derived from a biological (usually non-plant) source (Table 46.1). Vaccines are also considered here, even though they are constituted from whole attenuated/inactivated organisms as well as antigen subunits. The biopharmaceutical class of drugs includes proteins and peptides, nucleic acids and carbohydrates, all chemical components that exist in nature (Fig. 46.1). There are few carbohydrate drugs derived from biological sources, with heparin being the most well known, and while carbohydrate therapeutics are now being studied more intensely, they will not be treated in detail here because of the paucity of commercial products and information available. Other biopharmaceuticals include cell types such as the emerging area of transplanted stem cells and engineered tissues.
Since the 1990s, there has been huge growth in the area of biopharmaceuticals and this growth is predicted to continue, largely fuelled by the difficulty in identifying small molecule candidates for the diseases that still endure less than optimal treatment regimens or no treatments at all. The biopharmaceuticals market is dominated by three classes of drugs: protein and peptide hormones, e.g. insulin; monoclonal antibodies, e.g. trastuzumab and vaccines, e.g. the influenza and the diphtheria – pertussis – tetanus (DPT) vaccines. Other biopharmaceuticals include haematopoietic factors such as erythropoietin (epoetin); cytokines, e.g. the interferons; and enzymes such as the pancreatic enzyme, pancrealipase. Gene therapeutics currently constitute only a tiny fraction of the market, in essence limited to three authorized products – Gendicine, marketed in China, Rexin G, marketed in the Philippines and Glybera, recently approved in Europe. Ribonucleic acid gene silencing agents, commonly known as small interfering RNAs (siRNAs), while an area of active scientific endeavour, are yet to be marketed.
One aspect that unites these therapeutic molecules is that they are still administered mainly using a syringe and needle, despite the extensive efforts that have been made to deliver these compounds by non-parenteral means. Biopharmaceuticals are largely high molecular weight molecules (>5,000 daltons), with the exception of the short peptides and they suffer from instability problems, either on storage or after being administered. These properties make the administration of these compounds by non-parenteral means problematic at best and frequently impossible.
This chapter will serve as an introduction to the delivery issues associated with key members of this class of drugs, as well as an introduction to the established and emerging delivery solutions.
Protein and peptide drugs
Introduction
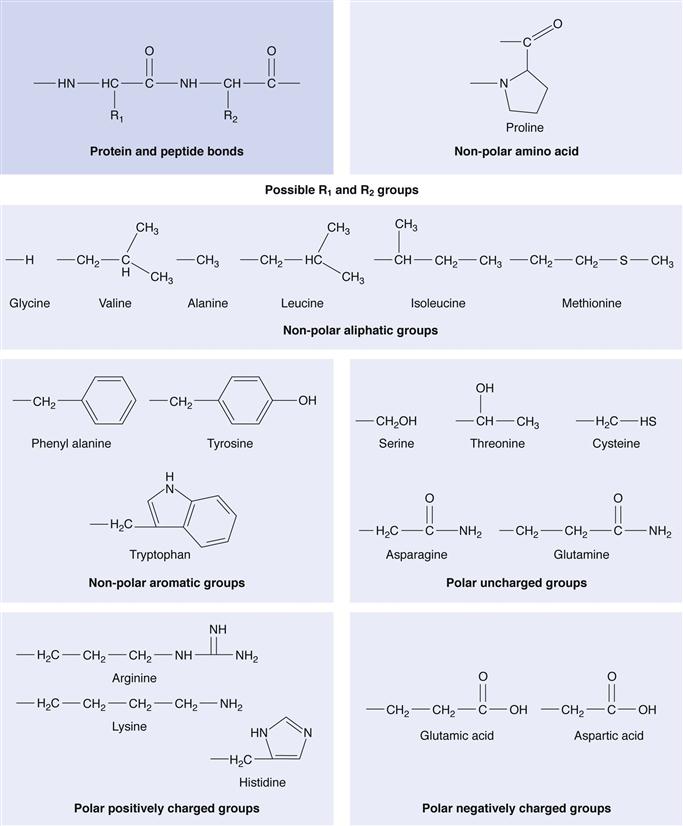
Proteins are composed of individual amino acids linked by amide bonds. The 20 known amino acids (Fig. 46.1) are the constituent parts of proteins. Amino acids are chiral compounds and amino acids of biological origin are L-amino acids. Peptides differ from proteins mainly in the number of amino acids contained within each molecule. The amino acid residue distinction between peptides and proteins is not exact: peptides are generally defined as having less than 50 amino acids, while proteins usually contain hundreds of amino acids and have a tertiary (folded) structure. However, insulin with 51 amino acids is defined as a peptide.
Endogenous proteins are synthesized in the cell in an amino acid sequence that is defined by a specific nucleotide base pair sequence. Following the synthesis of the protein, there is post translational modification, including glycosylation and protein folding to give the functional three-dimensional structure. Endogenous bioactive peptides are also synthesized within the cell and are normally the result of cleavage of larger proteins to give the peptide active. There are a number of therapeutic classes of proteins on the market (Table 46.1). The peptide therapeutic classes mostly comprise peptide hormones, such as insulin and calcitonin as well as endogenous peptide analogues, such as goserelin.
Production
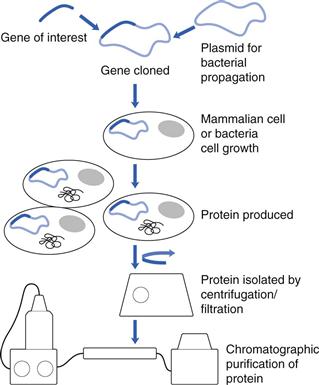
Protein drugs are produced in mammalian cells, e.g. the Chinese Hamster Ovary (CHO) cell line; bacteria, e.g. Escherichia coli (E. coli) or yeast cells (Fig. 46.2). The gene of interest is transfected into the cells and the cells are grown in a bioreactor. The protein product is isolated by cell lysis and centrifugation/filtration and the protein is purified using chromatographic techniques. Protein yields are an important determinant of the efficiency of the process, with yields in CHO cells of about 5 g L−1. In an effort to improve yields, new cell lines have been introduced, e.g. the PER. C6 cell line, created by the transfection of human retinal cells with the Adenovirus 5 E1A gene. This cell line gives high cell densities (160 X 106 cells mL−1) and antibody yields as high as 25 g L−1.
Whilst the usual sources of proteins are mammalian and yeast cells, others such as animal sources are also being investigated. For example, transgenic animals have been used to produce human antithrombin in goats’ milk. Cheap plant sources of proteins could revolutionize the biotechnology industry, lowering production costs, reducing drug prices and would have a positive effect on patient access to these therapeutic agents. The feasibility of producing high value protein products in plant species has been recently demonstrated with the production of trastuzumab in the Nicotiana benthamiana specie using viral gene expression systems.
Peptides, such as insulin, are produced using the above described recombinant techniques, whereas shorter peptide chains (less than 50 amino acids) are synthesized using relatively expensive and laborious solid phase synthetic techniques.
Once proteins are produced using recombinant means, the product is characterized to establish its identity and purity using the analytical techniques outlined in Table 46.2.
Table 46.2
Protein characterization techniques
| Analytical technique | Physical/chemical basis of the technique | Protein characterization |
| Sodium dodecyl sulphate (SDS) PAGE analysis – a qualitative evaluation of molecular weight against molecular weight markers | Separates proteins according to their electrophoretic mobility and molecular weight | Provides a qualitative evaluation of protein molecular weight and detects proteolysis and dimerization impurities |
| Isoelectric focusing | Separates proteins according to their charge, as a function of pH | Characterizes the homogeneity of the protein product |
| Capillary zone electrophoresis | Separates proteins according to hydrodynamic radius, friction and charge | Characterization of glycosylation patterns |
| Size exclusion chromatography plus light scattering | Separates proteins according to hydrodynamic radius and measures molecular weight | Determines protein molecular weight |
| High performance liquid chromatography | Separates molecules according to polarity and quantifies protein levels | Demonstrates protein purity and used to analyse impurity levels |
| Spectroscopic methods Nuclear magnetic resonance (NMR) spectrometery Ultraviolet absorption spectroscopy Circular dichroism infrared (CDIR) spectroscopy | Records electronic and molecular transitions in response to electromagnetic energy | Primary structure from NMR spectroscopy Reference material characteristics (e.g. from the IR fingerprint region) Secondary structure from CD and IR spectroscopy |
| Liquid chromatography – mass spectrometry (LC-MS) | Separation according to hydrophobic – hydrophilic balance and the detection of mass fragments | Peptide mapping |
| Differential scanning calorimetry | Measures the enthalpy change of protein thermal transitions (e.g. protein folding) | Data on protein stability |
| Epitope mapping | Identifies antibody binding sites on a protein – these may be linear peptide epitopes or conformational epitopes arising from protein folding Requires synthesis of the epitope followed by binding studies | Measures efficacy and immunogenicity |
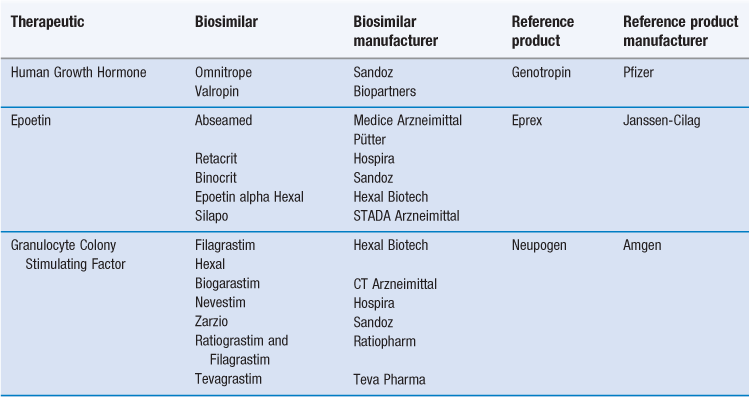
Production processes are normally proprietary and as a consequence, it is impossible, once the protecting patents have expired, for other companies to produce exactly the same therapeutic protein with identical glycosylation patterns without access to the original bioreactor procedures. This realization has led to a new category of medicine – the ‘follow-on biologic’ made by a separate manufacturer after patent expiry. Such follow-on products are also known as ‘biosimilars’ or ‘biobetters’. They must be comparable with respect to a number of key indicators in order to be considered biosimilars of reference marketed products. A number of biosimilars have been introduced and the biosimlars currently available in Europe are listed in Table 46.3.
Delivery issues
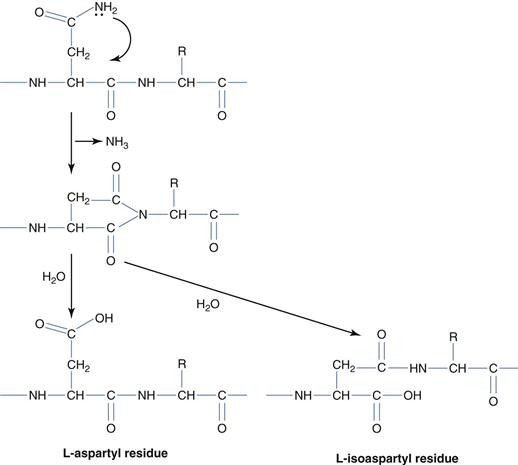
The delivery issues surrounding protein and peptide drugs are divided into two main categories: a) maintaining stability on storage and b) the optimization of in vivo efficacy. Storage stability issues may be classified according to the chemical and physical origins of protein instability. Of the chemical origins of protein instability – deamination (Fig. 46.3) is arguably the most intensively studied and occurs with the most frequency. Deamination is the result of base catalysed hydrolysis of asparagine (usually) and glutamine side chain amides to give aspartic acid and glutamic acid respectively. The reaction mechanism, which proceeds via the 5-membered cyclic ring, is the more prevalent mechanism, and the loss of ammonia on cyclization effectively makes the process irreversible. On deamination of asparagines, both aspartic acid and L-isoaspartic acid are formed.
Peptide bond hydrolysis is also a source of instability and this occurs at aspartic acid and tryptophan sites and the hinge region of antibodies. Racemization of amino acids to convert from the L-form to the non-natural D-form also occurs at aspartic acid residues. Additionally, base-catalysed nucleophilic attack mediated amine terminal cyclization, to give diketopiperazine groups; occasionally with the loss of the first two amino acids or an amine terminal attack on the side chain of glutamic acid groups to produce a cyclic pyroglutamic acid residue, are both promoted at basic pH. Oxidation of proteins by reactive oxygen species occurs at histidine, methionine, cysteine, tyrosine and tryptophan residues and disulphide bond formation between cysteine residues is also a source of protein chemical instability. Base-catalysed protein degradation may be controlled by storage at acid pH (pH = 3–6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree