Fig. 10.1
Low grade urothelial neoplasia (Voided fresh urine, Millipore filtration, high mag.)
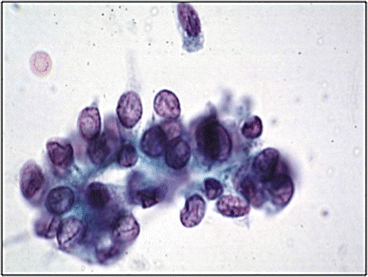
Fig. 10.2
Low grade urothelial neoplasia (Voided fresh urine, CS, high mag.)
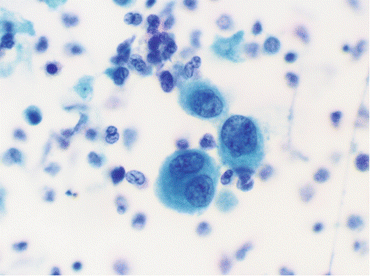
Fig. 10.3
Atypical urothelial cells (Voided fresh urine, SP, high mag.)
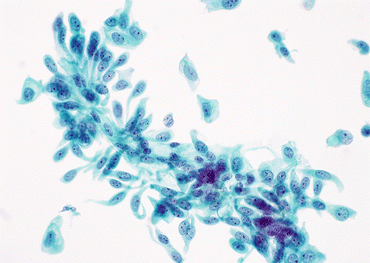
Fig. 10.4
Cercariaform urothelial cells from a case of LGUN (Voided urine, TP, high mag.)
Millipore Filtration (EMD Millipore, Billerica, MA)
The specimen is collected fresh (i.e., without added alcohol as a preservative), and concentrated by conventional centrifugation. The supernatant was discarded, and the cell button was resuspended by being vortexed in a few mL of balanced electrolyte solution. The resuspended cells were processed by membrane filtration (i.e., filtered at 100-mmHg negative pressure on a 5-μm pore size, 47-mm-diameter Millipore filter) that was rinsed with balanced electrolyte solution, and fixed in situ by adding 95 % ethanol. The cells were kept wet (i.e., not allowed to air-dry), and the filter was transferred to a Petri dish of alcohol. Subsequently, the filter preparation was taken through a modified Pap stain and coverslipped. Membrane filtration is labor-intensive, and is rarely used these days. Nonetheless, it is capable of recovering well-flattened cells that display their nuclear chromatin usefully.
Cytocentrifugation (Shandon Cytospin, Thermo Fisher Scientific, 81 Wyman Street, Waltham, MA)
The specimen is collected fresh (i.e., without added alcohol as a preservative), and concentrated by conventional centrifugation. The supernatant is discarded, and the cell button is resuspended by being vortexed in a few mL of balanced electrolyte solution. Place the specimen volume in the sample chamber (DO NOT EXCEED 0.25 mL) using a 1 mL graduated disposable pipette. Carefully layer 0.25 mL of 2 % carbowax over the specimen using a 1 mL graduated disposable pipette by dripping the carbowax down the side of the chamber. Secure and lock the chamber in place per the manufacturer’s instructions. Urine specimens are centrifuged at 1000 rpm for 8 min, “High Acceleration.” After cytocentrifugation, immediately wet fix in 95 % Ethanol. Slides can be Pap-stained by the routine Non-Gyn method.
SurePath ® (Becton Dickinson and Company, Franklin Lakes, NJ)
Preparation per manufacturer’s instructions.
The specimen is collected fresh (i.e., without added alcohol as a preservative), and concentrated by conventional centrifugation. The supernatant is discarded. The cell pellet may be handled in two ways. It may be resuspended in 10–15 mL of CytoRich Red Preservative; allowed to stand for at least 30 min; centrifuged; decanted; resuspended in 10 mL of balanced salt solution (BSS); centrifuged; decanted and vortexed, or it may be resuspended into 10–15 mL of SurePath Gyn Preservative Fluid or CytoRich Clear Preservative; vortexed; allowed to stand for at least 30 min; centrifuged; decanted and vortexed. The pelleted cells are then placed on the SurePath PrepStain device where they are resuspended, mixed, and transferred to a PrepStain Settling Chamber mounted on a SurePath PreCoat slide. The cells are sedimented by gravity, then stained using a modified Papanicolaou staining procedure. The slide is cleared in xylene or a xylene substitute and coverslipped. The cells are presented within a 13-mm-diameter circle
ThinPrep ® (Hologic, Bedford, MA)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


