Cytologic Evaluation of Menstrual Disorders and Hormonal Abnormalities
PRINCIPLES
The established approach to the evaluation of ovarian function and endocrine disorders in the woman is based on serial biochemical analyses of hormones, such as estrogen, progesterone, luteinizing hormones and their metabolites (Albertson and Zinaman, 1987). More recently, the analysis of hormonal substances that participate in embryonal development of gonads has contributed still further to the clarification of endocrine disorders. For example, the measurements of the müllerian-inhibiting substance, a hormone that promotes the involution of the müllerian duct and thus enhances the development of male characteristics, allowed the discrimination between male children with a congenital absence of testes (anorchia) and undescended testes (Lee et al, 1997). In children suspected of other congenital abnormalities, molecular-genetic analyses may be performed. For example, in children with congenital adrenal hypoplasia, who may also display underdevelopment of gonads, a mutation of the responsible gene located on the X chromosome could be documented (Merke et al, 1999). Many additional such examples could be cited. In women who suffer from menstrual disorders and abnormalities of the ovarian cycle, the biochemical analyses can be effectively supplemented by the old-fashioned endometrial biopsies, or studies of endocervical mucus (Lotan and Diamant, 1978). In addition, the cervicovaginal smear may sometimes provide useful information and has the advantage of being easy to obtain, rapidly evaluated, and inexpensive. The cytologic approach is particularly valuable if laboratories specializing in endocrine analysis are not readily available. The principle
of the cytologic hormonal analysis is simple. The degree of maturation of the squamous epithelium of the female genital tract depends on steroid hormones, mainly estrogen. Estrogen receptors are present in the squamous epithelium of the uterine cervix and vagina, particularly in the basal cells (Press et al, 1986; Kupryjanczyk and Moller, 1988), and are expressed more strongly during the proliferative phase of the cell cycle than during the secretory phase, accounting for the observed changes in epithelial maturation. Therefore, the quantitative relationship of squamous cells of varying degree of maturity in a cervicovaginal smear may serve as an index of the hormonal status of the female. In some cases of congenital abnormalities, the analysis of sex chromatin bodies (Barr bodies) in the same smears may yield valuable information.
of the cytologic hormonal analysis is simple. The degree of maturation of the squamous epithelium of the female genital tract depends on steroid hormones, mainly estrogen. Estrogen receptors are present in the squamous epithelium of the uterine cervix and vagina, particularly in the basal cells (Press et al, 1986; Kupryjanczyk and Moller, 1988), and are expressed more strongly during the proliferative phase of the cell cycle than during the secretory phase, accounting for the observed changes in epithelial maturation. Therefore, the quantitative relationship of squamous cells of varying degree of maturity in a cervicovaginal smear may serve as an index of the hormonal status of the female. In some cases of congenital abnormalities, the analysis of sex chromatin bodies (Barr bodies) in the same smears may yield valuable information.
It is beyond the scope of this book to discuss all the variants and metabolites of the steroid hormones. For the sake of simplicity, and because of their impact on cervicovaginal cytology, the key hormones and their activity to be discussed are estrogen, progesterone, and androgenic (masculinizing) hormones.
MECHANISMS OF FORMATION OF STEROID HORMONES
The main function of steroid hormones in the woman is to induce ovulation and prepare the uterus for pregnancy. The regularity with which this process occurs during the childbearing age of a normal woman is astounding and as yet not fully understood. As was summarized in Chapter 8 and Figure 8-18, the formation of steroid hormones by the ovary is governed by polypeptide hormones of pituitary origin (follicle-stimulating hormone [FSH] and luteinizing hormone [LH]). The ovary synthesizes the steroid hormones, which act on the endometrium, squamous epithelium, and other target cells in the uterus.
Mechanisms of Action of Pituitary Hormones on Ovarian Target Cells
The first sequence, which has for its purpose the formation in the ovary of various steroid hormones such as estrogen and progesterone from cholesterol, may be briefly summarized as follows:
Pituitary peptide hormone binds to the receptors on the membrane of ovarian cells with endocrine function, stimulating an increased activity of a common mediator, adenylate cyclase. Adenylate cyclase stimulates the synthesis of 3′5′-adenosine monophosphate (cyclic AMP or cAMP) from adenosine triphosphate (ATP).
cAMP activates the appropriate protein kinase leading to the formation of an enzyme, cholesterol esterase, and other enzymes involved in the biosynthesis of steroid hormones.
Mechanisms of Formation of Steroid Hormones in the Ovary
The cholesterol esterase leads to an increased accumulation of free cholesterol as a precursor for steroid biosynthesis.
Free cholesterol is transformed by enzymes into steroid hormones in the smooth endoplasmic reticulum of the appropriate ovarian cells. Estrogen is produced by the follicular cells of the maturing ovarian follicles. After ovulation, progesterone is produced by cells of the corpus luteum. For a recent review of the mechanisms of formation of steroid hormones in the pituitary and the gonads, see Adashi and Hennebold (1999).
Effect of Steroid Hormones on Target Cells
Ovarian steroids, such as estrogen and progesterone, interact with cells in the endometrium and the squamous epithelium of the cervix and vagina and the smooth muscle of the uterus. This sequence may be briefly summarized as follows:
The steroid hormone binds to a specific cytoplasmic receptor protein in the cells of the target organ and enters the cytoplasm. As has been recently reported, there are at least two types of estrogen receptors with complex patterns of function (McDonnell and Norris, 2002).
Steroid protein complex enters the nucleus and binds to the DNA specific receptor. Messenger RNA is formed.
Messenger RNA enters the cytoplasm, binds to ribosomes and leads to the synthesis of specific proteins, such as enzymes or structural proteins, that are expressed in the cytoplasm of the target cell.
Nuclear receptors for estrogen and progesterone have been identified and sequenced. Recent investigations shed light on the regulation of hormonal receptors in various target cells (McDonnell and Norris, 2002). This led to the development of specific antibodies that can be used to visualize the presence of these receptors in the nucleus by means of immunofluorescence or an immunocytochemical approach using the peroxidase-antiperoxidase reaction that forms visible precipitates. The precipitates can be measured by image analysis and related techniques (Bacus et al, 1988). This system of steroid receptor identification and quantitation has been extensively used in the assessment of mammary carcinoma (see Chap. 29), but its applicability to the cells of the female genital tract has been limited.
The effects of estrogen and progesterone on the endometrium and endometrial cells are discussed in Chapter 8. In this chapter, the effect of these and other hormones on the squamous epithelium of the female genital tract will be discussed.
THE EFFECT OF STEROID HORMONES ON SQUAMOUS EPITHELIUM OF THE FEMALE GENITAL TRACT
Smear Patterns
Naturally occurring estrogen, or the parenteral administration of estrogen or its natural or synthetic substitutes
in adequate amounts, produces a rapid and complete maturation of the normal squamous epithelium of the female genital tract with a resulting preponderance of mature superficial squamous cells in smears. The effect takes place regardless of the prior hormonal status, except during pregnancy. Conversely, complete atrophy of the squamous epithelium of the vagina and cervix may be equated with complete absence of estrogenic activity. However, there are no reliable data linking intermediate degrees of maturation of the squamous epithelium with the action of a specific hormone or hormones. Thus, partial or incomplete maturation of the squamous epithelium may have various causes, such as an inadequate or low supply of estrogen and its derivatives; an effect of antagonistic hormones, such as progesterone or androgenic hormones; or the effect of some of the widely used estrogen agonists, such as tamoxifen; or to a combination of these and probably other factors. The fact that surgical castration does not necessarily lead to complete atrophy of the squamous epithelium strongly supports the possibility that extragenital hormonal factors, such as adrenal hormones, are capable of influencing the squamous epithelium of the genital tract of the female.
in adequate amounts, produces a rapid and complete maturation of the normal squamous epithelium of the female genital tract with a resulting preponderance of mature superficial squamous cells in smears. The effect takes place regardless of the prior hormonal status, except during pregnancy. Conversely, complete atrophy of the squamous epithelium of the vagina and cervix may be equated with complete absence of estrogenic activity. However, there are no reliable data linking intermediate degrees of maturation of the squamous epithelium with the action of a specific hormone or hormones. Thus, partial or incomplete maturation of the squamous epithelium may have various causes, such as an inadequate or low supply of estrogen and its derivatives; an effect of antagonistic hormones, such as progesterone or androgenic hormones; or the effect of some of the widely used estrogen agonists, such as tamoxifen; or to a combination of these and probably other factors. The fact that surgical castration does not necessarily lead to complete atrophy of the squamous epithelium strongly supports the possibility that extragenital hormonal factors, such as adrenal hormones, are capable of influencing the squamous epithelium of the genital tract of the female.
It is obvious, therefore, that the patterns of squamous cells should be interpreted cautiously in terms of endocrine status or therapeutic indications. Unfortunately, rigid diagnostic standards in this area of genital cytology are difficult to establish and, consequently, the literature is replete with contradictory statements. This is well illustrated by the problem of cytologic evaluation of pregnancy at term, discussed in Chapter 8. Nonetheless, in specific clinical situations, discussed below, endocrine cytology is a valuable guide to diagnosis and treatment.
Hormonal Cytology in Various Age Groups
Evaluation of the endocrine status of a menstruating woman during the childbearing age belongs among the most difficult tasks in diagnostic cytology. There is considerable variation in the smear patterns from one patient to another, even if matched for age and menstrual history. Furthermore, there is considerable variation from cycle to cycle in the same patient. Daily variations and even variations between simultaneously obtained smears may occur. Even the mere effect of smear-taking may influence the pattern of the following smear by increasing the vascularity of the epithelium.
However, hormonal evaluation of the smears may be of substantial assistance in situations associated with amenorrhea or other significant disturbances of the menstrual cycle and may be of value in determining the time of ovulation for artificial insemination or in vitro fertilization (see below).
Evaluation of the endocrine status in prepubertal and postmenopausal patients is an easier and more rewarding task than during the childbearing age. In a patient whose baseline smear shows complete atrophy, an increased maturation of the squamous epithelium is easy to assess. Still, even the procedure of smear-taking may reduce the dryness of the vagina and result in a less atrophic smear pattern. In the absence of atrophy, some of the problems described above for women in the childbearing age may also preclude an accurate cytologic evaluation of hormonal effect.
The Influence of Factors Other Than Hormonal on Endocrine Evaluation of Smears
Several factors other than endogenous or exogenous hormones may influence the status of the squamous epithelium.
Inflammatory Processes
As is described in Chapter 10, inflammatory processes, particularly Trichomonas vaginalis infestation, may result in increased maturation of squamous cells in postmenopausal women. Histologic and colposcopic evidence suggests that increased vascularity of the epithelium may be a factor in this process. Other inflammatory processes, particularly coccal bacterial infections, may obscure the smear pattern because of pus formation. Therefore, it is advisable to forego any attempts at estimation of maturation of squamous cells in the presence of a marked inflammatory process.
Cancer
In postmenopausal women with carcinoma of the uterine cervix or endometrium, abnormally high levels of maturation of the squamous epithelium may be observed. Although in endometrial cancer this may represent a hormonal effect, in cervix cancer the effect is probably due to inflammation (Cassano et al, 1986).
Cytolysis
Cytolysis caused by Lactobacillus (the Döderlein bacillus) or related organisms may destroy squamous cells in sufficient numbers to preclude any reasonable estimation of level of maturation (see Chaps. 8 and 10). The effects of hormones and other drugs precluding the assessment of the hormonal status are discussed below.
Radiotherapy, Surgery and Other Interventions
Radiotherapy to the vagina or cervix exercises marked immediate and long-term effects on smear patterns, as discussed in Chapter 18. It is evident that radiotherapy, with its protracted and significant influence on the biology of the squamous epithelium, restricts the possibility of subsequent estimation of hormonal activity by smears. Surgery, cauterization, and other forms of treatment of diseases of the vagina or cervix also preclude proper hormonal evaluation until the healing has become complete—usually no fewer than 6 weeks after the procedure.
TECHNIQUES IN THE CYTOLOGIC EVALUATION OF THE HORMONAL STATUS
Vaginal Smears
Several conditions must be fulfilled before a successful hormonal evaluation of the squamous epithelium may be undertaken.
There must be absence of inflammation or cytolysis.
There must be no recent medication, either topical or systemic, especially with compounds known to affect the squamous epithelium of the lower genital tract.
There must be no history of radiotherapy or recent surgery to the vagina or cervix.
An adequate baseline investigation must have been performed in menstruating women. This should include daily smears during at least one and preferably two complete cycles, or their chronologic equivalent. In nonmenstruating patients, two or three smears may suffice.
The smears should be obtained from the proximal portion of the lateral wall of the vagina, care being taken to avoid contamination with material from the adjacent cervix. Soost (1960), in an elaborate study, confirmed that this is the area of the vagina most accurately reflecting the hormonal status. The squamous epithelium of distal vagina or of the cervix shows a lesser response to hormonal stimulation.
Although routine cervicovaginal smears are less accurate for purposes of hormonal evaluation, their use cannot be unequivocally condemned because the patterns of maturation of squamous cells may provide useful information either in the presence of a marked estrogenic effect (dominance of mature squamous cells in smears) or complete absence thereof (atrophic smear pattern).
Urocytograms and Other Methods
A number of observers, starting with Papanicolaou (1948), and subsequently Lencioni et al (1969, 1972), Haour (1974), and O’Morchoe (1967), reported results of hormonal evaluation with the use of an “urocytogram,” which is the evaluation of squamous cells in smears obtained from the sediment of voided urine. It is not completely clear whether the squamous cells in the urinary sediment are a contaminant with cells of vaginal origin or whether they reflect the presence of squamous epithelium of vaginal type that may be observed in the bladder trigone of many women (see Chap. 22). The urinary sediment has the advantage of easy collection without the necessity of a gynecologic examination and is particularly useful in the evaluation of some endocrine disorders in infants and children.
Other methods of endocrine evaluation include smears obtained from the inner aspect of labia minora of the vulva, as suggested by Tozzini et al (1971).
In congenital disorders in which X chromosome may be affected, the evaluation of sex chromatin bodies is conveniently performed in vaginal smears just as in scrape smears of the oral mucosa. Although this is not the primary topic of this chapter, it must be mentioned here that endometrial biopsies offer important information on ovulation and disturbances of the menstrual cycle. For further discussion of this topic, see Chapter 13.
OBJECTIVE EVALUATION OF HORMONAL PATTERNS
The Indices of Squamous Cells
To confer numerical reproducibility upon hormonal evaluation, several indices defining the status of the squamous cells in a smear have been advocated.
The Karyopyknotic Index (KI)
The karyopyknotic index expresses the percentile relationship of superficial squamous cells with pyknotic nuclei to all mature squamous cells. Usually, 200 to 400 consecutive cells in three or four different fields on the smear are evaluated. According to Pundel (1966), in a normally menstruating woman, the peak of KI usually coincides with the time of ovulation and was estimated at 50% to 85%. Variation from patient to patient is considerable. Schneider et al (1977) found a statistically significant correlation between KI and plasma estradiol levels as measured by radioimmunoassays.
The Eosinophilic Index (EI)
The eosinophilic index expresses the percentile relationship of mature squamous cells with eosinophilic cytoplasm to all mature squamous cells, regardless of the status of the nucleus. The procedure is similar to that described for the karyopyknotic index. Often the simple Shorr’s stain (see Chap. 44) is used in preference to Papanicolaou stain. Pundel (1966) reported that in a normal menstruating woman, the peak of EI coincides with the peak of KI and may reach 50% to 75% at the time of ovulation.
The Maturation Index (MI)
The maturation index, first described by the Czech investigator, Nykliček in 1951, expresses the maturation of the squamous epithelium as a percentile relationship of parabasal cells to intermediate cells to superficial cells. The count should be performed on single cells. Cell clusters must be avoided. For example, in a normal menstruating woman at the time of ovulation, an MI of 0:35:65 would indicate that the smear contained no parabasal cells, 35% of intermediate cells, and 65% of superficial cells. A postmenopausal patient with marked atrophy would have a MI of 90:10:0, indicating marked preponderance of parabasal cells. Reyniak et al (1971) found good correlation between the MI and endometrial biopsies for staging of the menstrual cycle. Estrogen-type smear corresponded to proliferative endometrium in 83% of cases, and the premenstrual type of smear corresponded to secretory endometrium in 88% of cases. On the other hand, Schneider et al (1977) found a poor correlation between MI and plasma estradiol levels.
The Maturation Value
Meisels (1967) suggested that a specific numerical value be attached to the three principal subgroups of the squamous cells—a value of 1.0 to superficial squamous cells, a value of 0.5 to intermediate cells, and a value of 0.0 to parabasal cells. The maturation value (MV) would be expressed by
multiplying the percentage in each cell category by its assigned value. For example, the MV for the two patients discussed above under MI would be as follows:
multiplying the percentage in each cell category by its assigned value. For example, the MV for the two patients discussed above under MI would be as follows:
Patient 1 with MI 0:35:65 (normal menstruating woman at time of ovulation)
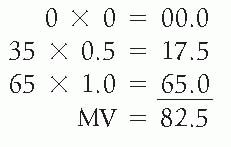
Patient 2 with MI 90:10:0 (woman with postmenopausal atrophy)
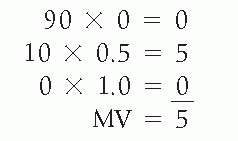
This system gives a single figure from zero to 100 to express the hormonal status of the patient and, thus, offers advantages for computerized handling of data. An MV of 100 indicates a pure population of superficial squamous cells, MV of zero indicates a pure population of parabasal cells. For menstruating normal women, the MV is between 50 and 95; for women with varying degrees of atrophy of squamous epithelium, the MV is below 50.
Other Indices
The folded-cell index represents the relationship of mature superficial or intermediate squamous cells with folded cytoplasm to all mature squamous cells. The crowded-cell index represents the relationship of mature squamous cells lying in clusters of four or more cells to all mature squamous cells.
The reader is referred to several papers by Wied et al (1968, 1992) listed in the bibliography for additional information on the various indices.
Critique of Indices
In an extensive study, Cordoba (1964) pointed out that any indices based on a single smear are not reliable. Even several smears obtained on the same day yield an error of 20%, if 1,000 cells are evaluated. This critique is valid in my experience. Particularly vulnerable is the eosinophilic index, because it is known that even a short exposure to air may significantly increase the proportion of cells with eosinophilic cytoplasm. The most reasonable of the indices is the maturation index and its derivative, the maturation value, since it gives an idea of the make-up of the squamous epithelium. However, to obtain results that would withstand a critical statistical analysis, at least 500 single cells, dispersed in four quadrants of the smear, should be counted, surely a time-consuming procedure.
Alternative Ways of Reporting Hormonal Status
It has been my practice to base the evaluation of the maturation of the squamous epithelium on an overall visual impression gained during the routine screening of smears. Dr. George Wied has suggested the term estimogram for this procedure. This simplest of methods has not failed in revealing major abnormalities of smear patterns. By comparing the current smear pattern with original baseline smears, a good appreciation of changes in smear pattern may be gained. Small variations in smear pattern have no diagnostic meaning but may strongly influence the indices and thus give a false impression of hormonal “effects.” The reporting of smears based on this overall visual impression is always given in reference to age, menstrual history, and possible clinical significance. Some examples follow:
Patient age 35: “Midcycle smear pattern—consistent with functioning ovaries.”
Patient age 52: “Absence of maturation of squamous cells consistent with menopause.”
Patient age 25: “Absence of maturation of squamous cells—abnormal for age.”
Patient age 60: “High level of maturation of squamous cells not consistent with clinical menopause. It is assumed that this patient is not receiving estrogens or other drugs that may account for this smear pattern.”
DETERMINATION OF THE TIME OF OVULATION FROM CERVICOVAGINAL SMEARS
A precise determination of the time of the ovulation is important in artificial insemination and in in vitro fertilization, which incidentally is also valuable in animal husbandry. Hormonal patterns of cervicovaginal smears were used as a guide for embryo transfer in in vitro fertilization with fair results (Bercovici et al, 1988). Boquoi and Hammerstein (1969) reported that vaginal hormonal cytology correlated poorly with hormonal patterns of urinary steroid hormone secretion in patients in whom ovulation was induced with clomiphene citrate. The use of the cervicovaginal smears to establish the time of ovulation or the status of the endometrium has been of limited reliability




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


