Chapter 30 Cutaneous metastases and Paget’s disease of the skin
Cutaneous metastases
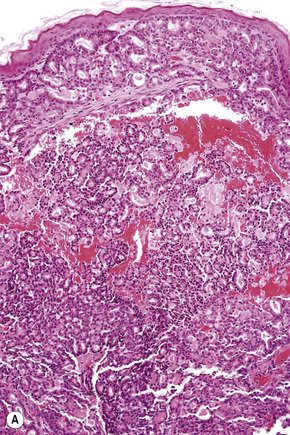
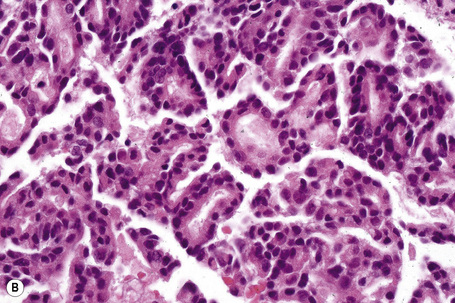
Secondary involvement of the integument by a malignant tumor may represent a metastatic phenomenon (such as following vascular or lymphatic emboli) or occur as a direct consequence of contiguous spread through tissue spaces or lymphatic and vascular channels (Figs 30.1, 30.2).1,2 In addition, tumor deposits may be accidentally implanted during surgical procedures.3,4 Rarely, cutaneous metastasis may be the presenting feature of a visceral malignancy.5–7
Clinical features
Incidence, primary sites and chronology of presentation
The more frequent sites for secondary tumor deposits are lymph nodes, liver, lungs, adrenals, brain, bone, ovaries, and kidneys. The skin is a relatively uncommon site for presentation of metastasis; recorded frequencies have varied from 0.7% to 9% of all cases of metastatic malignant disease.2,7–9 A recent meta-analysis including over 20 000 patients with carcinoma, and excluding melanoma, found the incidence of cutaneous metastases to be 5.3%.10 Considering the fact that the skin is the largest organ in the body, it is still unclear why this relatively low incidence of metastasis from internal malignancy is observed. As initially described by Paget in his ‘seed and soil’ hypothesis, the tumors preferentially metastasize to those organs with an intrinsically favorable environment.11 It is therefore possible that the skin may provide the proper environment for colonization and survival of only a few types of tumors. Recently, chemokines and their receptors have been shown to be involved in tumorigenesis and homing mechanisms for metastatic cells. The chemokine receptor CCR10 has been demonstrated to be involved in cutaneous metastatic melanoma and may mediate melanoma survival in the skin.12,13
Cutaneous metastases present most commonly a few months or years after the primary tumor has been diagnosed.14 There are rare reports of metastatic tumors arising decades after the diagnosis of the primary neoplasm.15 Cutaneous metastases develop most commonly at the same time as internal metastases.16 Less frequently, a metastasis is diagnosed concurrent with the primary tumor or represents the initial manifestation of the disease.8 In a study by Brownstein and Helwig, skin metastases were the presenting sign of the disease in 37% of men and in 6% of women.8 This reflects the fact that although metastatic breast carcinoma represents the most common skin metastasis in women, currently breast cancer is usually diagnosed fairly early in the course of the disease as opposed to many other internal malignancies. Cutaneous metastases from some primary sites appear more likely to be the first sign of the disease. These include lung cancer (60% of cases), renal cancer (53% of cases), and ovarian cancer (40% of cases), but the incidence of skin metastasis as the initial presenting symptom is likely falling dramatically from these historical levels with earlier clinical detection of internal disease in contemporary medicine. Although cutaneous metastases may present at any age, the greatest incidence is in the fifth to seventh decades.
The relative frequencies of the underlying primary neoplasms largely reflect the incidence of the various tumors that occur in humans.9 In Western communities the most common sources of cutaneous metastases in males include the lungs (24%), large intestine (19%), melanoma (13%), squamous carcinoma of the oral cavity (12%), kidney (6%), stomach (6%), and esophagus (3%) (Fig. 30.3).8 In females the primary tumor site is most often the breast (69%), while other important sources include the large intestine (9%), melanoma (5%), ovaries (4%), and uterine cervix (2%) (Fig. 30.4).8 With the increased incidence of cigarette smoking in females, carcinoma of the bronchus is of growing importance. Much less common primary sites have been reported including thyroid, adrenal, endometrium, urinary bladder, and pancreas.9,17 Prostate carcinoma, despite its high incidence in men, is among the least common primary site to result in cutaneous metastases.
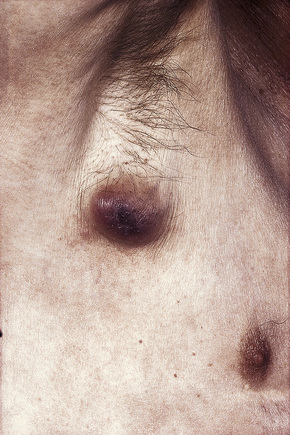
Fig. 30.3 Metastatic bronchial carcinoma: the lung is the commonest source for cutaneous metastases in males.
By courtesy of R.A. Marsden, MD, St George’s Hospital, London, UK.
In a study by Lookingbill, melanoma was the most common source of metastatic disease in the skin in men and the second most common source for women (Fig. 30.5).9 A study from India has shown that the most common primary sites of cutaneous metastasis are the lung and esophagus in men and the breast and ovaries in females.18
With the exceptions of leukemia and lymphoma, cutaneous metastases from underlying malignancies in children are very rare. However, around 50% of malignant cutaneous tumors in children represent metastatic disease.19 Interestingly, and in contrast to adults, cutaneous metastases represent the first manifestation of the disease in up to 84% of cases.
The most common childhood tumors metastasizing to the skin are rhabdomyosarcoma and neuroblastoma, reflecting the incidence of these tumors in this age group (Fig. 30.6).20 Neuroblastoma is most often seen in neonates. In this age group, neuroblastoma presents with skin metastases in up to 32% of cases.21
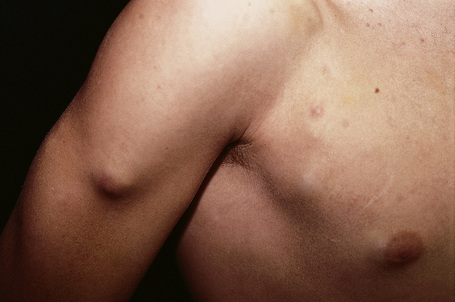
Fig. 30.6 Metastatic neuroblastoma: there are multiple metastases on the chest wall and arm.
By courtesy of Drs José María Ricart and Amparo Marquina Vila, Hospital Peset, Valencia, Spain.
Rare tumors metastasizing to skin in children include osteosarcoma, choriocarcinoma, Ewing’s sarcoma, malignant rhabdoid tumor, melanoma, paraganglioma, and nasopharyngeal carcinoma. Choriocarcinoma presenting as a metastatic tumor in a neonate is associated with a primary placental tumor, which can also spread to the mother.22 As with adults, central nervous system tumors only exceptionally spread to the skin in children.23 In the vast majority of children with metastatic carcinoma in the skin, the source of the primary is almost invariably determined after thorough and comprehensive investigation.24 An unknown primary is truly exceptional.
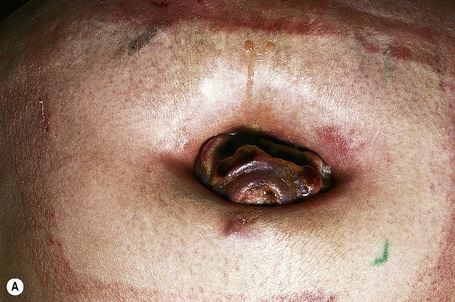
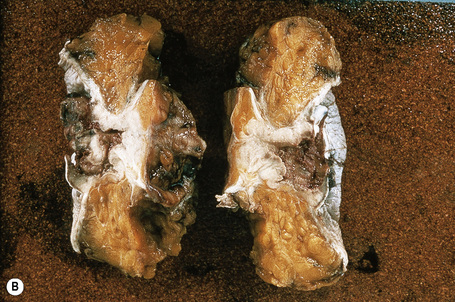
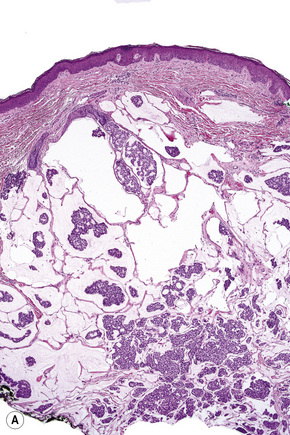
Although not always the rule, the location of a cutaneous metastasis often reflects the site of the underlying tumor.25 The anterior chest, abdomen, head, and neck regions are the most common sites for metastatic tumors. Multiple sites of cutaneous metastases (involving two or more anatomic regions) have also been reported. Metastases to the skin of the face and neck are most often associated with a squamous carcinoma of the oral cavity, although lung, kidney, and breast may also represent rare sources of such metastases. Carcinoma of the breast most frequently spreads to the anterior chest wall, while bronchial and lung tumors tend to metastasize to the chest wall and upper extremities (Fig. 30.7). Gastrointestinal tumors usually involve the anterior abdominal wall but may present in other areas such as the face and scalp;26 pelvic neoplasms show a predilection for the perineal region. Primary tumors of the prostate and bladder can spread to the penis or scrotum.27–31 Intra-abdominal tumors, including those of the stomach, colon, ovary, endometrium, urinary bladder, fallopian tube, prostate, cervix, and pancreas, sometimes metastasize to the skin of the umbilicus to form the so-called Sister Mary Joseph’s nodule (Fig. 30.8).32–45 Rarely, a metastatic breast carcinoma or mesothelioma can present as a Sister Mary Joseph’s nodule. Umbilical metastases represent the first manifestation of disease in about 14% of cases.41
Metastases to the limbs are uncommon, most often resulting from spread of primary cutaneous melanoma. Sometimes these present in a subungual location. Subungual metastases can also complicate carcinoma of the stomach, lung, esophagus, kidney, colon, and breast.46–50 Subungual metastases from a choriocarcinoma have also been reported.51
Melanoma and neoplasms associated with vascular invasion (e.g., renal cell carcinoma, thyroid follicular carcinoma, and choriocarcinoma) lack such a tendency towards regional localization (Figs 30.9, 30.10).
Some tumors are characterized by a predilection to metastasize to certain sites.52 For example, ocular melanoma selectively spreads to the liver. In addition to skin, lung, bowel, and brain are also common sites for melanoma metastasis.52
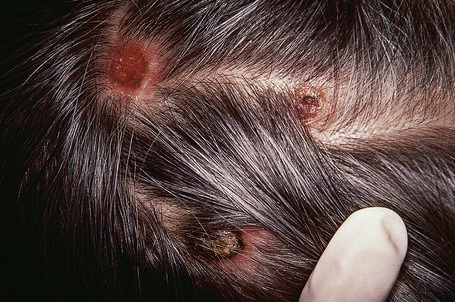
Involvement of the scalp is not uncommon, amounting for up to 5% of all metastases.53–55 Sometimes it may represent a primary manifestation of the underlying malignancy.1,8,56–58 In a large series of malignant tumors of the scalp from Taiwan, metastatic disease accounted for 12.8% of cases.59 The most common primary site in both men and women in this series was the lung. Tumors most likely to spread to the scalp include those of the breast, lung, colon, stomach, and kidney (Figs 30.11, 30.12). Scalp metastases are particularly common in renal cell carcinoma. A plaque of alopecia is the usual manifestation but occasional cases of metastatic breast carcinoma without alopecia have also been described.56,59
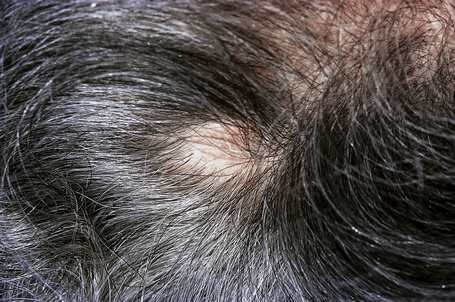
Fig. 30.11 Metastatic carcinoma of breast: scalp lesion associated with focal alopecia.
By courtesy of M. Shaw, MD, St Thomas’ Hospital, London, UK.
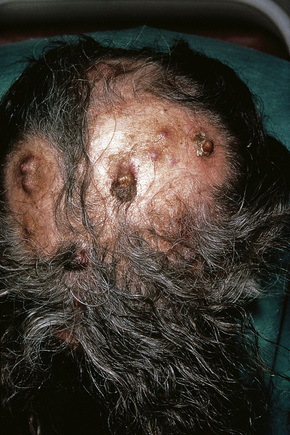
Fig. 30.12 Metastatic carcinoma of kidney: scalp lesions associated with numerous nodules and alopecia.
By courtesy of R.A. Marsden, MD, St George’s Hospital, London, UK.
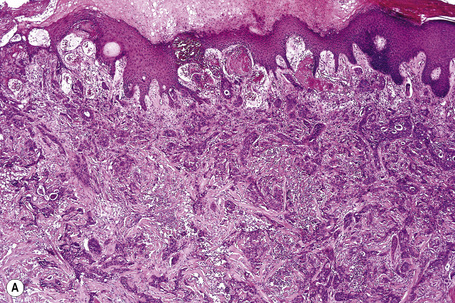
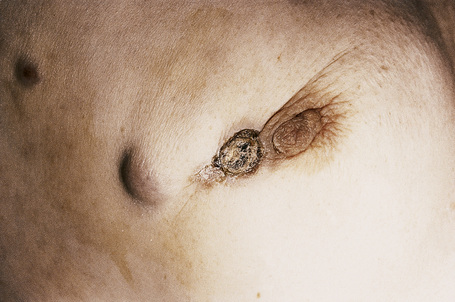
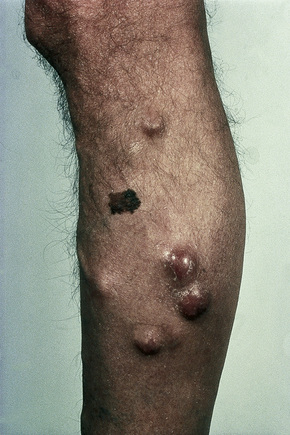
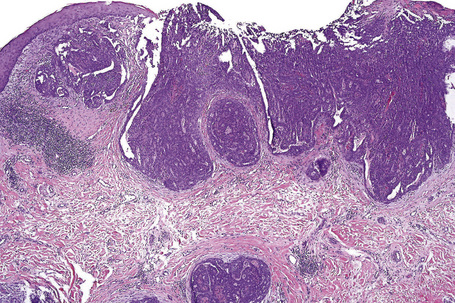
Of the various patterns of cutaneous metastasis, the most common is development of a nodule or group of nodules (frequently in a surgical scar) typically painless, rapidly growing, freely mobile, and often flesh colored (Figs 30.13–30.16).8 Most lesions are less than 3 cm in greatest diameter. Unless traumatized, they rarely ulcerate. Other patterns of cutaneous metastases include micropapules, plaques, and lesions simulating scars. Scarlike lesions can be seen in metastases from the breast, stomach, lung, and kidney.60
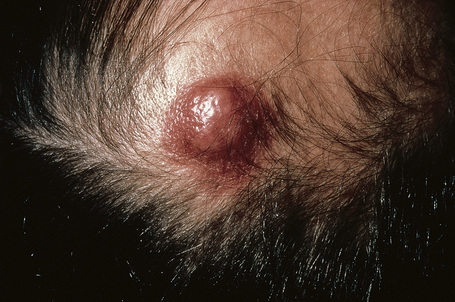
Fig. 30.13 Metastatic carcinoma: most metastases present as a flesh-colored or erythematous nodule.
By courtesy of Drs José María Ricart and Amparo Marquina Vila, Hospital Peset, Valencia, Spain.
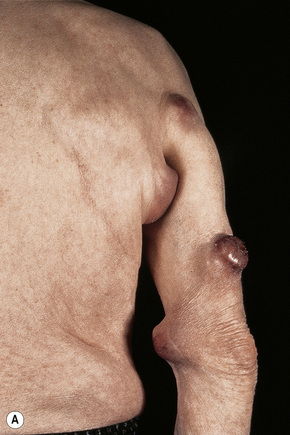
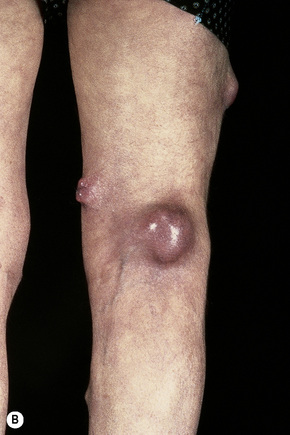
Fig. 30.16 Metastatic melanoma: tumor deposits are present on (A) the arm and (B) the leg.
By courtesy of the Institute of Dermatology, London, UK.
The clinical appearance of some metastases mimics a number of benign dermatological conditions such as erythema annulare centrifugum, contact dermatitis, cellulitis, kerion, chancre, follicular cyst, and condyloma.61–65 Other clinical presentations include zosteriform and elephantiasiform lesions, facial lymphedema (Fig. 30.17), and hemorrhagic blisters.66–73 Zosteriform metastases have occurred with many malignancies including melanoma, squamous cell carcinoma, eccrine porocarcinoma, angiosarcoma, and carcinomas of the breast, ovary, colon, bronchus, tonsil, prostate, and bladder, and transitional carcinoma of the renal pelvis.68,74–76
Cutaneous metastasis can also mimic primary cutaneous tumors such as pyogenic granuloma, cylindroma, keratoacanthoma, blue nevus, Kaposi’s sarcoma, angiosarcoma, and melanoma.77–85
Hemorrhagic cutaneous metastatic nodules usually imply a primary renal cell carcinoma, thyroid follicular carcinoma or choriocarcinoma. Pigmented metastases can mimic a melanoma.86,87 The pigmented nature of the tumor is most likely due to epidermotropic metastasis that causes damage to the epidermis and subsequent pigmentary incontinence.
Exceptionally, metastases to benign cutaneous lesions such as nevi have been documented.88,89 Unusual presentation of metastases includes spread to skin with radiation changes and to sites of surgical procedures.90 The latter include scars following removal of abdominal organs or mastectomy sites, percutaneous biopsies or fine needle aspirates, pericardiocentesis, thoracocentesis, and sites of implantation of biliary catheters.60,91–96
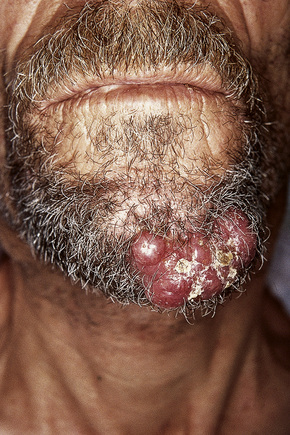
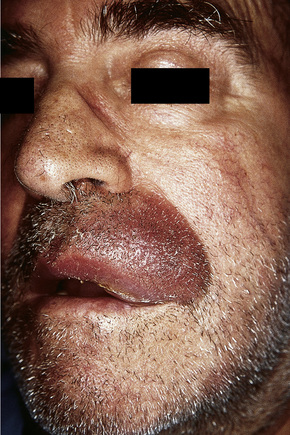
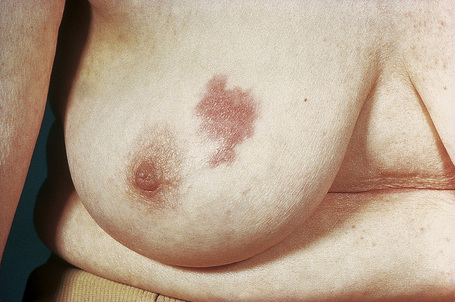
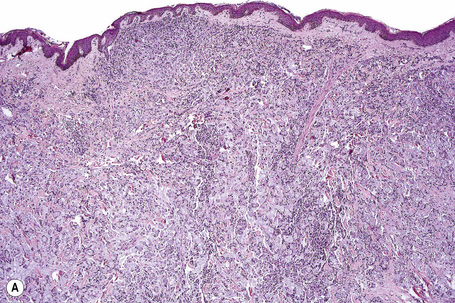
There are other modes of presentation of cutaneous metastases which are particularly but not exclusively related to breast cancer. Inflammatory cutaneous metastasis (carcinoma erysipelatoides) is fairly rare and results from massive lymphatic obstruction by tumor with associated edema.97–100 It presents as a tender, erythematous, warm plaque (Fig. 30.18) located in most cases on the anterior chest wall (particularly in females with pendulous breasts). Clinically, it may be misdiagnosed as cellulitis or erysipelas, but theoretically, at least, it may be distinguished by the absence of fever and leukocytosis. Inflammatory cutaneous metastases have also been described with carcinoma of the prostate, lung, large bowel, pancreas, stomach, kidney, ovary, tonsil, parotid, uterus, esophagus, melanoma, and bladder.101–109
Also associated with a poor prognosis are breast metastases presenting on the anterior chest wall as violaceous telangiectatic papules, vesicles or nodules resembling lymphangioma circumscriptum.110–112 This is known as carcinoma telangiectaticum and it may also be seen rarely with lung carcinoma. The lesions are sometimes purpuric and hemorrhagic and resemble a vasculitic process.113 Other manifestations of breast cancer are peau d’orange, representing dermal edema due to obstruction of superficial cutaneous lymphatic vessels and carcinoma en cuirasse, an intensely sclerotic plaque of tumor (Fig. 30.19).1,97–100,114,115
Metastatic breast carcinoma infrequently presents in the inframammary crease in a characteristic pattern mimicking dermatitis or resembling a primary epithelial tumor including basal cell and squamous cell carcinoma.116–118
A clinical variant that sometimes causes diagnostic confusion is the sclerodermatous (eburneous) metastasis usually associated with tumors having a dense fibrous stroma, such as carcinoma of the breast and pancreas.115 The lesion is usually nontender and nonpruritic, and is most often located on the scalp with associated alopecia, so-called alopecia neoplastica. The latter may be confused with lichen planopilaris, pseudopélade, lupus erythematosus, alopecia areata, keloid, and morpheaform basal cell carcinoma.8,119 Alopecia neoplastica has also been described in association with metastatic colonic carcinoma to the scalp.120
Neonates with metastatic neuroblastoma often present with multiple firm, blue–gray nodules in a typical appearance described as the ‘blueberry-muffin’ syndrome. This presentation, however, is not specific and can also be seen in lymphoma, leukemia, vascular tumors, and congenital infections.20
Rare cutaneous metastases
Some tumors have only very exceptionally been reported with skin metastases. Many of these primary tumors are themselves quite uncommon. These include glioblastoma, eccrine porocarcinoma or hidradenocarcinoma, pheochromocytoma, ameloblastoma, parotid adenocarcinoma (including malignant pleomorphic adenoma), chordoma, malignant thymoma, seminoma, Leydig cell tumor, papillary serous carcinoma of the uterus, ovarian malignant Brenner’s tumor, gallbladder adenocarcinoma, adrenocortical carcinoma (including a case presenting with cutaneous metastasis 30 years after the primary), transitional bladder carcinoma, hepatocellular carcinoma, carcinoid tumor, neuroendocrine tumors, mesothelioma, anaplastic carcinoma of the thyroid, medullary thyroid carcinoma, Sertoli cell tumor, salivary adenoid cystic carcinoma, and cholangiocarcinoma.9,36,55,79,121–146 More recently reported are metastases of myoepithelial carcinoma including one case in a child, well-differentiated fetal adenocarcinoma, adenocarcinoma of rete testis, malignant sacrococcygeal teratoma, prostatic small cell carcinoma, mucinous sweat duct carcinoma, adenocarcinoma arising in a longstanding nevus sebaceus of Jadassohn, small-cell neuroendocrine carcinoma of the uterine cervix, squamous cell carcinoma of the ureter, an exceptional case of acinic cell carcinoma of salivary gland presenting with cutaneous metastases 20 years after the primary, and a malignant blue nevus of the scalp with distant metastasis to the back.147–158
Sarcomas mainly involve the skin as a result of direct extension from the underlying subcutis or deeper soft tissues. Cutaneous metastatic sarcoma, which represent less than 3% of all cutaneous metastases, has been described in association with epithelioid sarcoma, leiomyosarcoma arising in different organs including the uterus and small intestine, chondrosarcoma, osteosarcoma including a postirradiation lesion, so-called malignant fibrous histiocytoma (undifferentiated pleomorphic sarcoma), giant cell tumor of bone, fibrosarcoma, rhabdomyosarcoma, gastrointestinal stromal tumor, myxofibrosarcoma, and epithelioid angiosarcoma.57,159–174 In the authors’ experience, metastatic leiomyosarcoma is most commonly encountered. A cutaneous metastasis derived from the chondrosarcomatous component of a metaplastic (sarcomatoid) breast carcinoma has also been described.175 A case of cutaneous metastasis from osteosarcoma has been reported as the first manifestation of disease.176 Atrial myxoma may embolize to the skin, particularly in the context of Carney complex.177–179
Prognosis
The presence of cutaneous metastases is usually (although not invariably) an ominous sign. The average survival is about 6 months after diagnosis.7 However, some patients may have long survival. This reflects both improvement in cancer therapy and the biological behavior of the individual tumor.180 For example, neuroblastoma in children can mature or undergo complete regression and patients with renal cell carcinoma can have a long survival following removal of the primary tumor and the metastases. In general, patients with cutaneous metastases from the upper digestive tract, upper respiratory tract, lung, and ovary have very poor survival.180 In a study involving 200 patients with cutaneous metastases, Schoenlaub and coauthors reported median survival times of 13.8 months for breast cancer, 2.9 months for lung cancer, and 6.5 months for all other cancers. 181
Histological features
Immunohistochemistry, electron microscopy and fine needle aspiration cytology
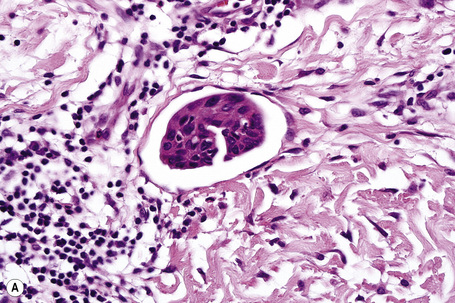
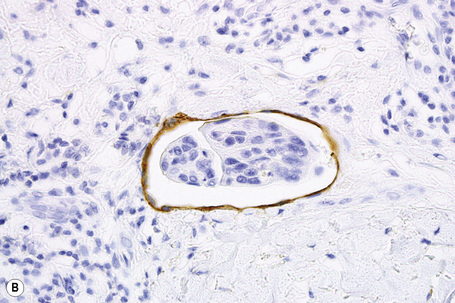
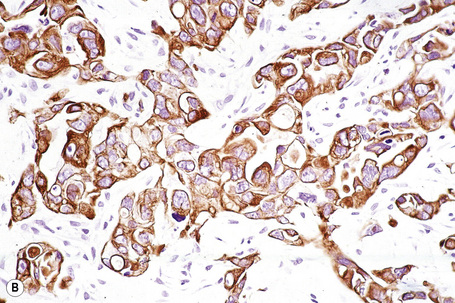
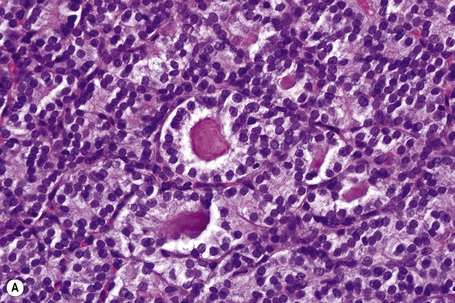
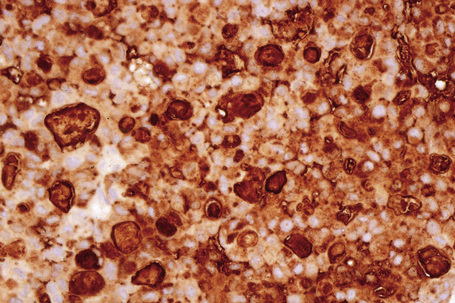
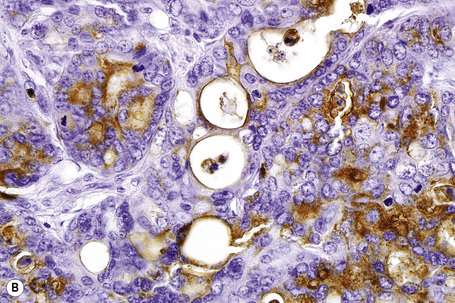
Immunohistochemistry is of particular value in distinguishing between various carcinomas, lymphomas, and pleomorphic sarcomas (Figs 30.20–30.24). With respect to metastatic adenocarcinoma, however, it is more difficult to accurately predict the likely site of the primary neoplasm.1,2 With a few specific exceptions, including thyroid (thyroglobulin), prostatic carcinoma (prostate specific antigen, prostatic acid phosphatase), and hepatocellular carcinoma (hepatocyte paraffin 1 and α-fetoprotein),3 if the precise diagnosis is not apparent after careful scrutiny of hematoxylin and eosin stained sections then it is less than likely that immunohistochemistry will be of definite help in resolving the problem.1,4
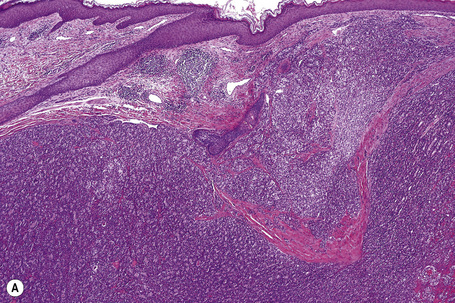
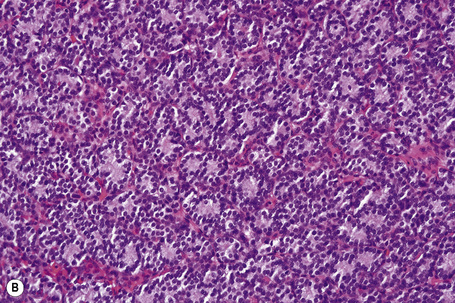
Fig. 30.21 Metastatic thyroid follicular carcinoma: (A) low-power view; (B) typical follicles are evident.
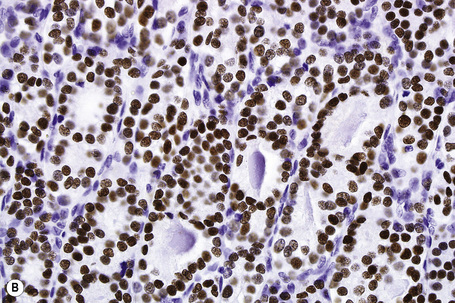
Distinction between primary cutaneous and metastatic neuroendocrine carcinoma is usually made by the presence of positive dotlike perinuclear staining for low molecular weight cytokeratin (CK) 20 and neurofilament protein expression in the former and absence of staining in the latter.5,6 Apart from the skin, cytokeratin 20 is only expressed consistently in neuroendocrine carcinomas of salivary gland origin.5 In contrast, bronchial neuroendocrine carcinoma expresses CK7. Although these findings are useful in the differential diagnosis in most cases, caution is recommended before basing a diagnosis on a single immunohistochemical result. Some cases of lung small cell carcinoma express CK20 and there are also reported cases of cutaneous neuroendocrine carcinoma (Merkel cell carcinoma) that are CK20-negative.7 The use of thyroid transcription factor (TTF-1) can also be of help in the distinction between a primary cutaneous and a metastatic bronchial neuroendocrine carcinoma: TTF-1 is negative in the former but present in the latter.8,9 However, occasional cases of Merkel cell carcinoma have been shown to be positive for TTF-1.10 Although CK20 is most commonly used, other cytokeratins, such as CAM 5.2, also label the tumor cells of primary cutaneous neuroendocrine carcinoma. Recently, a new marker has been described as useful in the distinction between pulmonary small cell carcinoma and Merkel cell carcinoma.10 MASH-1 (raised against Achaete-scute complex-like 1) is an important protein in the development not only of the brain but also of the neuroendocrine system. MASH-1 tends to be positive in pulmonary small cell carcinoma in up to 83% of cases but appears to be uniformly negative in Merkel cell carcinoma.10
Historically, gross cystic disease fluid protein 15 (GCDFP-15), estrogen receptor (ER), and progesterone receptor (PR) have been used as markers for breast differentiation. However, it is important to note that GCDFP-15, ER, PR, and Her2/neu have all been shown to be also expressed in cutaneous adnexal neoplasms, reflecting the close relationship between the sweat gland and the breast.11–15 For this reason, these markers should be used with caution if utilized in an attempt to distinguish primary cutaneous lesions from metastatic breast tumors.
Staining for other molecular weight keratins such as CK7 is of limited value in the differential diagnosis of metastatic carcinoma. Although this marker is absent from many carcinomas – including those originating in prostate, colon, kidney, and thymus – it is present in the majority of carcinomas arising in other organs and therefore its specificity is very limited.16 CK7 has been shown to be expressed in both primary cutaneous adnexal neoplasms and cutaneous metastases, mainly from the lung and breast.17 However, the pattern of CK7 labeling is distinctly different between the two categories: only focal in primary adnexal neoplasms, present in the inner luminal cells lining tubules and cysts, whereas there is strong and diffuse staining in metastatic adenocarcinomas.17 However, using CK7 and CK20 in combination with gross cystic disease fluid protein-15 (GCDFP-15) can be useful in determining whether a case of extramammary Paget’s disease is primary or metastatic.18–20 Primary perianal and extramammary Paget’s disease tends to be positive for CK7 and GCDFP-15 and negative for CK20, while metastatic rectal adenocarcinoma is usually negative for the former two markers and positive for the latter.18–20
Gastrointestinal metastasis to the skin can often be suspected by the presence of ‘dirty’ necrosis (intraluminal necrotic debris), commonly seen in primary and metastatic intestinal adenocarcinoma. The pattern of strong CK20 expression and lack of CK7 expression favors a metastasis from an intestinal primary tumor. Carcinoma originating in the hepatobiliary or upper gastrointestinal tract may be also CK7-positive and/or CK20-negative. CDX 2, a homeobox gene encoding an intestinal epithelial transcription factor, initially considered a specific marker for gastrointestinal tract origin has also been reported in other tumors, including ovarian mucinous tumors of the intestinal type, and rare tumors of the lung, bladder, and head and neck.21,22 Villin is an additional marker that may help to distinguish between colorectal (positive) and ovarian metastatic deposits (negative).23 It has been proposed that expression of HIK1083, a monoclonal antibody against alpha1,4-GlcNac-capped O-glycans, may be more useful than cytokeratin 20 in distinguishing between metastatic gastric carcinoma (positive) and primary sweat gland carcinomas (negative).24 This marker also tends to be negative in metastatic adenocarcinomas from other organs.
Cutaneous metastases from the lung often express CK7 and lack CK20. Additionally, approximately 75–85% of primary pulmonary adenocarcinomas express TTF-1.25,26
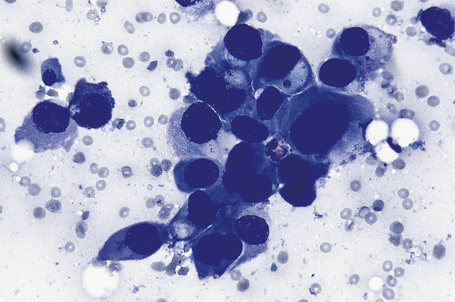
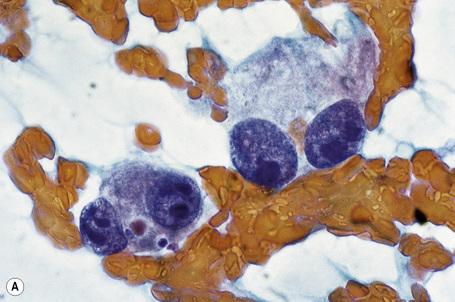
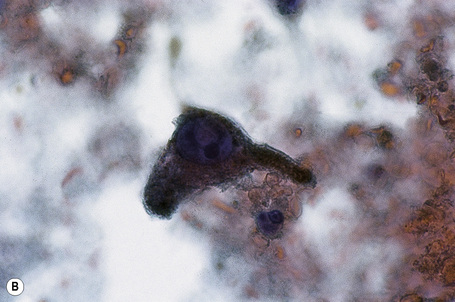
Over the past few decades, the enthusiasm for fine needle aspiration cytology has grown. The technique in many circumstances has proven to be a highly satisfactory alternative to more conventional excisional, punch or tru-cut biopsy specimens. In the dermatopathology setting, it is of particular value in assessing potential metastatic tumors, especially if the patient is unfit or unwilling to undergo further operative procedures (Figs 30.25, 30.26).27 Fine needle aspiration cytology is a fast, inexpensive, and effective method of confirming the diagnosis in the majority of cases. Where appropriate, the technique also permits the application of immunohistochemical investigations, fluorescent in situ hybridization (FISH), and PCR-based techniques.
Individual tumors
Squamous carcinoma
Metastatic squamous carcinoma reflects a wide variety of primary sites including the bronchus (30% of metastatic lung tumors), esophagus (less than 2% of esophageal cancers disseminate to the skin), oral cavity, larynx, and cervix.28–34 It may less frequently arise from a squamous carcinoma elsewhere on the integument, anus, vulva, penis, or vagina. Poorly differentiated metastases often cause difficulties, and multiple sections may have to be scrutinized carefully before foci of squamous differentiation (keratinization or intercellular bridges) are detected. An important point is that it is not possible to distinguish metastatic poorly differentiated squamous cell from transitional cell tumors in the absence of keratinization.
Usually, the metastatic tumor does not connect with the overlying epidermis. Exceptionally, however, a metastatic squamous cell carcinoma can present as an epidermotropic and/or folliculotropic metastasis, making distinction from a primary tumor very difficult. This phenomenon has been described in a metastatic laryngeal carcinoma, in a carcinoma from the lip, and in metastases from a primary cutaneous squamous cell carcinoma.35,36 Cytokeratin 5/6 can also be used for the diagnosis of squamous cell carcinoma, particularly in poorly differentiated tumors, but this does not allow a distinction between primary and metastatic neoplasms.37,38
The use of CK14 has been advocated as being helpful in distinguishing between a squamous cell carcinoma and an adenocarcinoma. Squamous cell carcinoma is frequently positive for this marker while adenocarcinoma is negative except in areas of squamous differentiation.39
Adenocarcinoma
Adenocarcinomatous deposits are by far the most common cutaneous metastasis, with the breast being the most frequent source (up to 23% of cutaneous metastases, most of which occur in women).28,31,40 Lung and large intestine are also important sources of metastatic adenocarcinoma.41,42 Other primary sites include the stomach, prostate, pancreas, endometrium, thyroid gland, ovaries, and endocervix.4,28,31,43–50
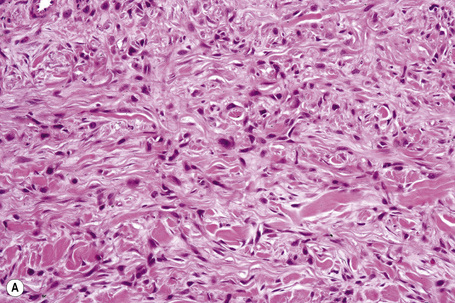
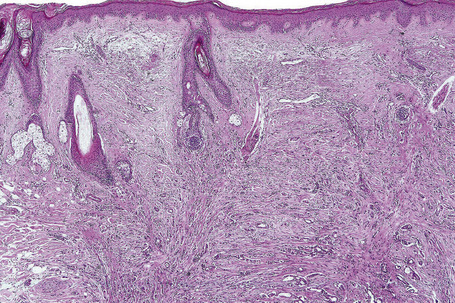
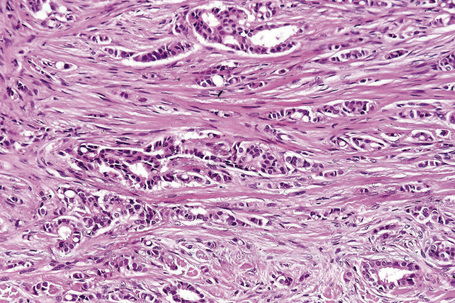
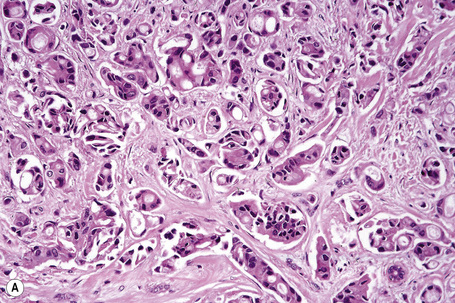
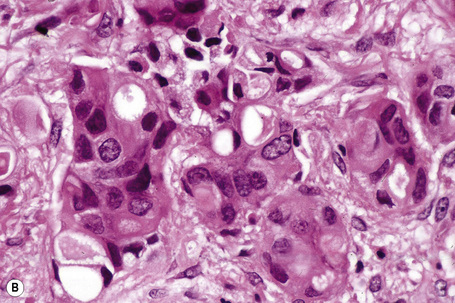
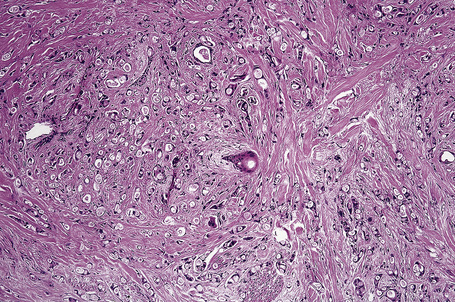
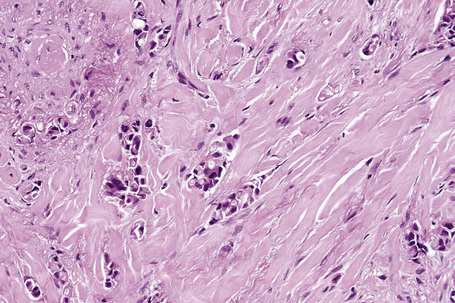
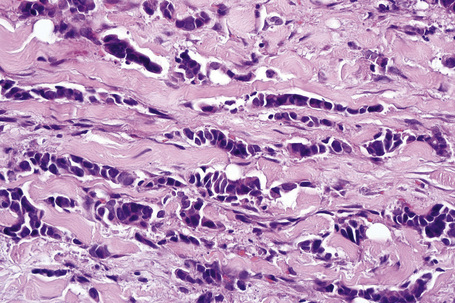
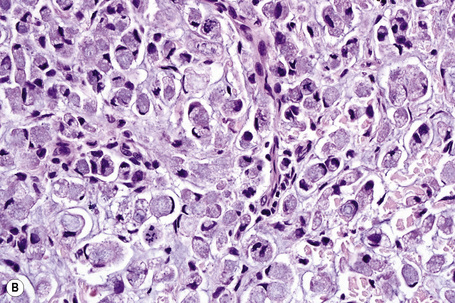
Metastases from carcinoma of the breast have a variety of appearances, depending to some extent on the nature of the primary tumor (Figs 30.27–30.31).51 Nodular deposits and carcinoma en cuirasse consist of a diffuse infiltrate of undifferentiated cells with hyperchromatic nuclei and minimal cytoplasm. Metastatic breast carcinoma to the eyelid is seen with some frequency and at that site the tumor cells sometimes have a prominent histiocytoid appearance.52 Rarely, metastatic adenocarcinoma of the breast, prostate, and colon can present with epidermotropism, and confusion with primary cutaneous carcinoma or superficial spreading melanoma may ensue.35,53 Exceptionally, metastatic breast carcinoma mimics a granular cell tumor or contains melanin pigment resembling a melanoma.54–56
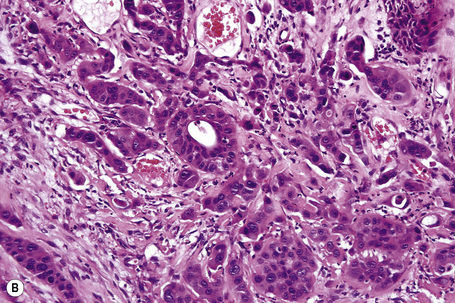
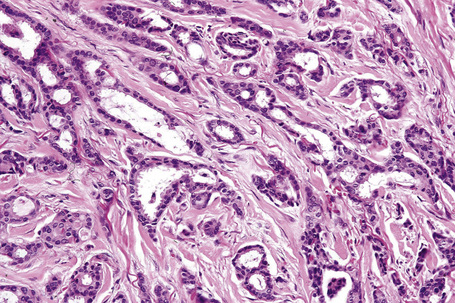
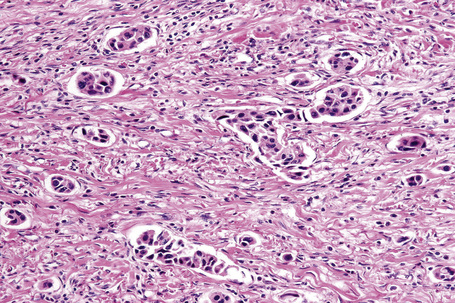
Typical of a breast metastasis, especially the lobular type, is the presence of linear dissection of tumor cells between adjacent collagen bundles (stacked-penny or ‘Indian-file’ appearance) (Fig. 30.32). Similar appearances may be seen with a number of other tumors including those of the prostate, stomach, and pancreas, and small cell carcinoma. Lymphoma and primary cutaneous neuroendocrine tumor may occasionally adopt an identical pattern. There may be obvious glandular differentiation, but it is unusual for this to predominate (Fig. 30.33). Inflammatory carcinoma is characterized by a widespread infiltration of the subepidermal and deeper lymphatic channels and, in the telangiectatic variant, vascular involvement may also be apparent (Figs 30.34, 30.35). A well-differentiated glandular architecture, with focal necrosis, is more typical of a primary tumor in the large intestine or rectum (Figs 30.36–30.39). In anaplastic variants, the features of the tumor cells may be indistinguishable from those of a high-grade lymphoma. Immunohistochemical techniques are often necessary to establish the correct diagnosis. Some CD30-positive anaplastic large cell lymphomas fail to express leukocyte common antigen (CD45). It is important to include a range of antibodies to both B- and T-cell antigens before excluding the diagnosis of disseminated lymphoma. It must also be remembered that nodal CD30-positive anaplastic large cell lymphoma not uncommonly expresses epithelial membrane antigen (EMA), which may therefore be a diagnostic pitfall for the unwary.
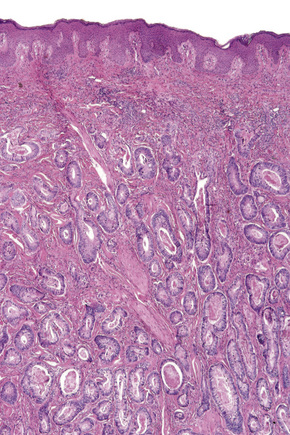
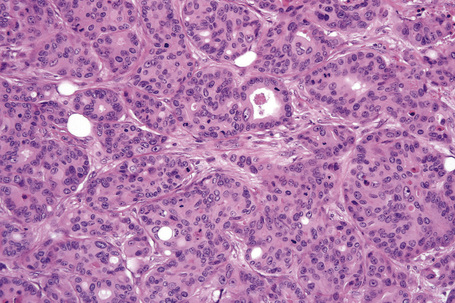
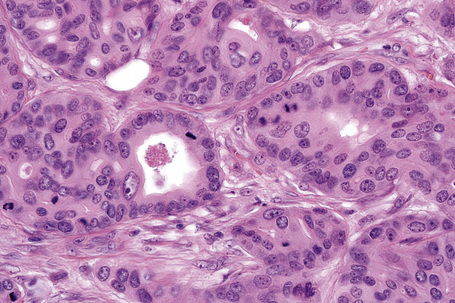
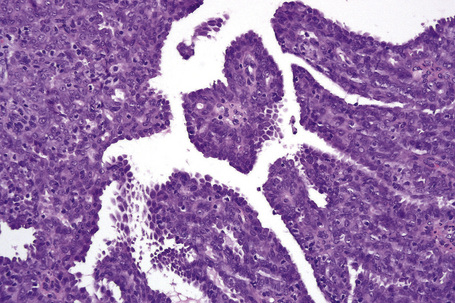
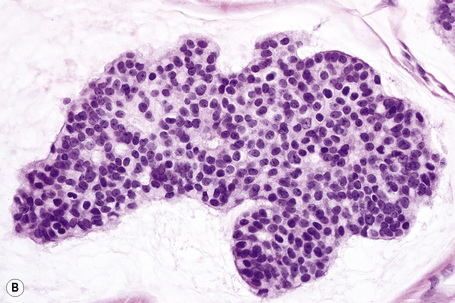
Metastatic tumors with a papillary growth pattern may reflect a number of different sites, including the colon, ovary, thyroid gland, stomach, and even the lung (Figs 30.40, 30.41).47–49 Certain distinguishing features sometimes permit differentiation between various papillary metastases. Concentrically laminated calcified psammomatous bodies may be conspicuous in both serous (ovarian) carcinoma and thyroid papillary carcinoma. The latter, however, may be further identified by colloid production, nuclear grooving, and characteristic ‘Orphan Annie’ nuclei.28 Intranuclear cytoplasmic pseudoinclusions are also sometimes a feature. The immunohistochemical demonstration of thyroglobulin production is confirmatory. Cutaneous metastasis of papillary thyroid carcinoma with prominent clear cell change may mimic a primary adnexal tumor such as a clear cell hidradenocarcinoma. A percentage of cases of metastatic papillary thyroid carcinoma show BRAF (V600E) mutations, a feature that is absent in follicular carcinoma of the thyroid and in hidradenocarcinoma, allowing distinction in difficult cases.57
It has recently been suggested that p63 can be used as a marker to distinguish primary adnexal tumors from metastatic adenocarcinoma in the skin: p63 is generally expressed in cutaneous adnexal tumors and is lacking in metastatic adenocarcinomas (breast, gastrointestinal tract, lung).17,58 It is important to note, however, that p63 immunohistochemical staining cannot be used to distinguish between primary and metastatic squamous cell carcinomas (whether of lung or head and neck origin) or metastatic urothelial carcinomas since it can be routinely identified in the normal basal cells of skin and other stratified epithelia, as well as in prostatic and respiratory epithelium.59,60 p63 is also a marker for myoepithelial cells of the breast.56
This observation has also been supported by other studies. Qureshi and coworkers studied 15 metastatic carcinomas to the skin including 14 adenocarcinomas and 1 urothelial carcinoma.17 Only one of the adenocarcinomas displayed partial p63 expression, and this was a poorly differentiated esophageal carcinoma. A more recent report by Sariya and coworkers found that when used as a single marker, a positive p63 stain had the highest sensitivity (96%) for primary adnexal tumors and a negative p63 stain had the highest positive predictive value for a metastasis.61 Kanitakis and coworkers also reported that the large majority (88.5%) of primary skin tumors express p63 while 89% of the metastatic tumors to skin are p63-negative.62 Ivan and coworkers have also demonstrated that strong p63 expression is retained in those rare cases of metastatic sweat gland carcinoma to other skin sites and lymph nodes.63 The use of p63 in a panel of immunohistochemical studies may therefore be of value in the differential diagnosis of metastatic adenocarcinoma to skin from primary cutaneous tumors showing ductal differentiation.
Another immunohistochemical marker that have been reported as useful in the differential diagnosis of cutaneous metastases from primary cutaneous adnexal neoplasms is CK5/6 which is expressed in the majority of primary cutaneous adnexal neoplasms, but only rarely in cutaneous metastases of internal adenocarcinoma.38 A further marker, podoplanin (D2-40), has also been reported as positive in primary adnexal tumors and not in metastatic tumors of various origin (lung, breast, gastrointestinal or genitourinary tracts).64 Calretinin, a calcium-binding protein expressed in mesothelial, epithelial, and stromal cells, has been found to be positive in metastatic tumors but also in some cases of primary cutaneous neoplasms. 61
Mucinous carcinoma
Mucinous carcinoma is characterized by compartments created by fibrous strands, which contain pools of mucin and floating nests of tumor cells. These usually have ample cytoplasm, centrally placed vesicular nuclei, and only mild cytologic atypia. Although mucinous carcinoma may sometimes arise as a primary tumor in the skin (most commonly as a slow-growing nodule on the head or neck of older men) on occasions it represents metastatic spread from sites such as the breast, stomach, colon, rectum, and pancreas (Fig. 39.42).65,66
Distinction between a primary and a metastatic tumor is usually not possible on histological grounds alone, although in a large case series, Kazakov and coworkers reported that the presence of an in situ component is particularly helpful in the diagnosis of primary cutaneous neoplasms. However, the absence of such a component does not exclude the diagnosis.66,67 Dirty necrosis has been reported as a constant histologic finding in metastatic adenocarcinoma of intestinal origin.66 Mucinous carcinoma found in the skin of the trunk usually represents metastatic disease from internal primary tumors.66
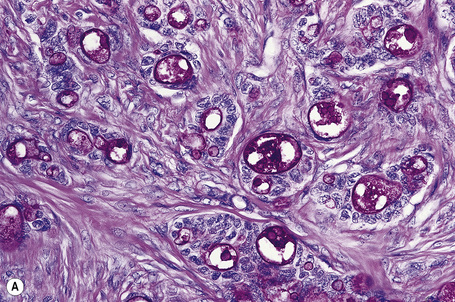
‘Signet ring’ metastases, in which intracellular mucin accumulation results in compression of the nucleus to the cell periphery, may be seen with gastric, large intestinal, and pancreatic primary tumors (Fig. 30.43). Rarely, they may also arise in the endocervix and gallbladder. Signet ring cell change may be seen in a number of cutaneous tumors including sweat gland carcinoma, melanoma, squamous cell carcinoma, and basal cell carcinoma.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree