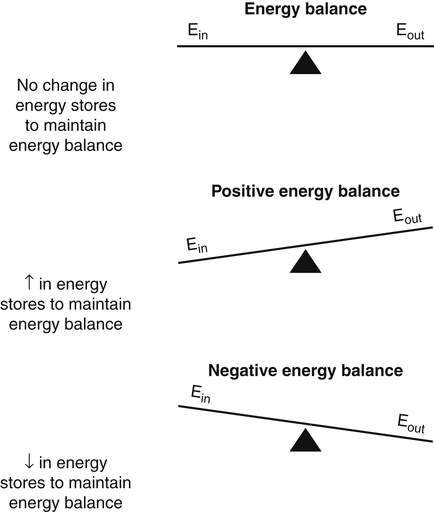
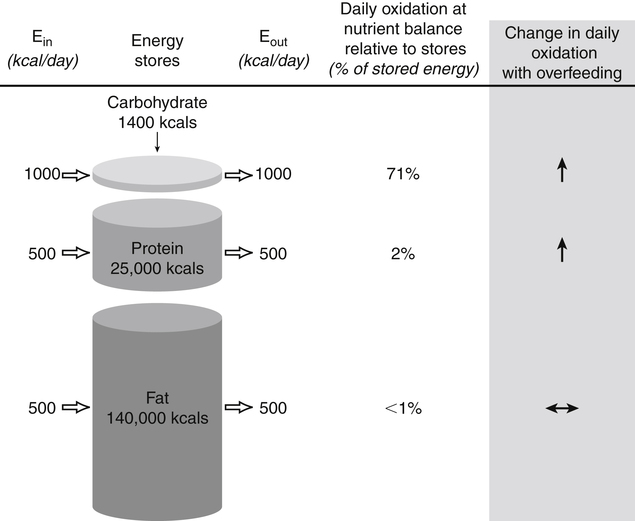
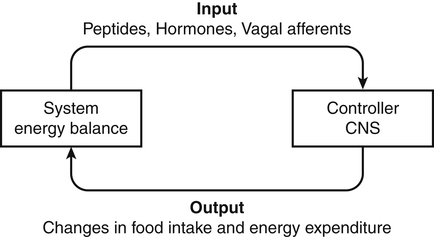
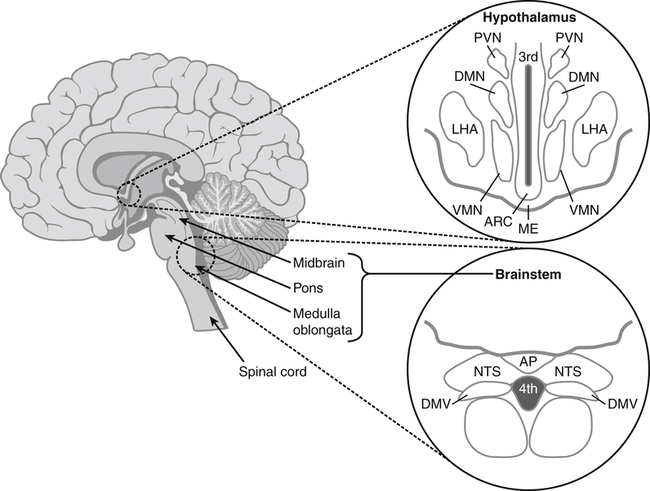
Darlene E. Berryman, PhD, RD, Brenda M. Davy, PhD, RD and Edward O. List, PhD∗ Energy balance, by definition, is a state in which energy intake and energy expenditure are equivalent over the period of observation such that there is no change in stores: Energy intake includes all the usable energy consumed in the form of carbohydrate, fat, protein, and alcohol and not lost in feces or urine. Energy expenditure includes the heat released for basal metabolism, physical activity, and thermic effect of food (TEF), as more fully explained in Chapter 21. As illustrated in Figure 22-1, when energy intake is not equal to energy expenditure, the energy stored in the body must be altered to balance the equation. That is, when energy intake exceeds energy expenditure, energy balance is positive and a net gain in body energy stores occurs. Conversely, when energy expenditure exceeds energy intake, energy balance is negative and a net loss in body energy stores occurs. Estimates of total body energy stores (dry weight) are shown in Table 22-1. When an individual is in energy balance, these stores remain stable. Energy to fuel basal metabolism and everyday activity is provided by energy-yielding nutrients in food. Short-term energy needs (e.g., between meals or during exercise) are met mainly by utilization of liver or muscle glycogen reserves and some fat. However, glycogen reserves and energy-yielding substrates in the circulation represent very small storage depots and normally are exhausted within 24 hours during a fast. During prolonged fasting or during energy restriction for weight loss, significant protein is also degraded and used for energy in addition to substantial catabolism of the fat depots. Most of the triacylglycerol stored in adipose tissue is available to the body during a prolonged fast, but not all energy contained in body protein is available for use as fuel. Body proteins serve important structural and functional purposes and therefore cannot be depleted without affecting survival of the organism. TABLE 22-1 Energy Stores in a 30-kg Child and a 70-kg Adult Man ∗Data from Rosenbaum, M., & Leibel, R. L. (1988). Pathophysiology of childhood obesity. Advances in Pediatrics, 35, 73–137. †Data from Cahill, G. F. (1970). Starvation in man. New England Journal of Medicine, 282, 668–675; Frayn, K. (1996). Metabolic regulation: A human perspective (pp. 78–102). London: Portland Press. All values are given as dry weights of triacylglycerol, protein, and glycogen that are available for consumption as fuel. These values are not the wet weights of tissue containing these fuels. In a practical sense, people do not eat “energy”; they eat nutrients in the form of food, and the nutrients are oxidized by the body to provide energy. The predominant energy-yielding nutrients in the human diet are the macronutrients: protein, carbohydrate, and fat. Alcohol, if ingested, is also a source of energy. Because there is little net conversion of either protein or carbohydrate into fat in humans unless large excesses of protein or carbohydrate are ingested, achieving balance between energy intake and energy expenditure generally means achieving a balance for intake and oxidation of each macronutrient (Flatt and Tremblay, 2004). As illustrated in Figure 22-2, nutrient and energy balance in the adult occurs when the intake of protein, carbohydrate, and fat (and alcohol) is equivalent to the body’s oxidation of each. When energy and nutrient balance are achieved, the following result: In contrast to the other macronutrients, fat can be stored in large amounts and fat intake is not tightly coupled to fat oxidation. As a consequence, fat stores serve as an energy buffer, and positive or negative energy balances are largely conditions of positive or negative fat balance. When excess calories are consumed in the form of a mixed diet, carbohydrate and protein that are not used to replace glycogen or body proteins will be preferentially oxidized. In contrast, any fat not oxidized will be stored in adipose tissue. For a given individual, the major factors that influence fat balance are overall caloric intake (i.e., the amount and composition of food eaten) and the total energy expenditure, especially the amount of physical activity (Peters, 2003). The set point theory is based on the recognition that humans tend to maintain stable body weight over long periods, and they defend this weight against conditions that would otherwise promote weight gain or loss. For example, when humans are overfed to force weight gain, upon cessation of overfeeding they spontaneously lose weight and return toward their starting condition. Although these individuals lose weight when no longer overfed, the weight loss often is not sufficient to return them to their exact starting point (Bouchard et al., 1996). Likewise, when individuals are subjected to a weight-reducing regimen for a prolonged period, they lose weight, but they rapidly regain the lost weight when the condition of negative energy balance is relieved (Sims and Horton, 1968). These observations in humans support the concept of a body weight set-point, such that the energy balance system in an individual is programmed to defend a particular body weight. Studies in experimental animals have also provided strong evidence of a physiological mechanism that defends a particular body weight under conditions of energy deficit or surplus (Keesey and Corbett, 1984). To alter this apparent body weight set-point, a change in the fundamental central nervous system (CNS) mechanisms regulating food intake and energy expenditure is required. For example, early animal studies showed that destruction of the ventromedial hypothalamus in the brain, the so-called satiety center, caused animals to overeat and reach a new higher body weight, which the animal then defended (i.e., a new set-point). Alternatively, destruction of the lateral hypothalamus, the so-called feeding center, resulted in reduced food intake, weight loss, and a new lower body weight or set-point that was defended. Recent studies have refined our understanding of the brain mechanisms affecting energy balance, and a number of specific hypothalamic neural networks and signaling systems that control different elements of energy intake, expenditure, and storage have been identified. Yet body weight often does not remain fixed throughout adult life, despite many years during which relative constancy is achieved. In an individual, changes in body weight over time can be in either direction. Therefore it could be more accurate to designate a particular body weight that an individual defends at a particular point in time as a settling point rather than a set-point. Settling points are determined by the different physiological, psychological, and environmental circumstances the individual may experience. For example, declining levels of physical activity encourage positive energy balance, weight gain, and thus a new, higher settling point (Hill et al., 2003). Similarly, an increase in body weight occurs in many women around the time of menopause (Sowers et al., 2007). Regardless of whether the set-point or settling point construct seems more appealing, the existence of a physiological control system that defends body weight cannot be denied based on the available evidence. Although body weight is defended in response to challenges that either decrease or increase energy stores, this defense appears to be asymmetrical. That is, the body’s defense against a gain in energy stores appears to be weaker than that protecting against a loss of energy stores. This is borne out by years of clinical experience in treating obese individuals; although a high percentage can achieve weight loss, only about 17% of individuals maintain weight losses of 10% or more (Kraschnewski et al., 2010). The bias in the energy balance control system toward promoting weight gain is also apparent in the growing prevalence of obesity among both adults and children. Today, more than one third of the U.S. population is obese, up from 25% just a decade ago, and an additional one third of the population is overweight (Flegal and Troiano, 2000; Flegal et al., 2010). The relatively weak defense against positive energy balance appears to exist whether the energy gain is provoked by increased energy intake, decreased physical activity, or both. In experimental overfeeding studies, it has been shown that the body has a limited capacity to burn off excess energy, and most of the excess is stored (Horton et al., 1995; Klein and Goran, 1993; Levine et al., 1999). Human eating behavior is a learned habitual behavior (Savage et al., 2007). Food intake in any given circumstance is influenced by the learning history of the individual as it relates to that circumstance. Learning begins in early childhood, at which time the child develops food preferences through experience with different foods. Properties of the food itself (e.g., orosensory properties), energy content and density, and the social and cultural context in which the food is consumed can all be associated with the metabolic effects of the food to yield a learned, conditioned response. The taste (e.g., sweet, sour), energy density, and variety of foods offered to children have been found to shape children’s food preferences. Infants and young children display an innate preference for the sweet taste, and young children demonstrate preferences for foods that are energy dense (Savage et al., 2007). Offering children a wide variety of foods to choose from increases the spectrum of foods they come to prefer. All of these factors appear to contribute to ensuring that the child consumes adequate energy and sufficient essential nutrients to support proper growth and development. As the child grows older, learned food acceptance patterns and eating habits are continually reinforced and extended, so that by adulthood there is a rich experiential background that affects eating behavior at any given meal. At any given eating occasion, the amount and composition of food an individual consumes are affected by the immediate context of consumption superimposed on the underlying biology (i.e., mechanisms controlling food intake and energy balance) and the learning history. As described earlier in the chapter, the two primary determinants of energy balance are energy intake and energy expenditure. Yet many factors influence energy intake and energy expenditure, both at individual and environmental levels. A list of some factors believed to influence energy balance behaviors is provided in Table 22-2. For example, the cost of food acquisition, the variety of foods present, food form (i.e., solid or liquid), energy density, portion size, and the number of other people present are just a few of the factors that affect short-term food and energy intake (Wansink, 2004; Rolls, 2009; Flood-Obbagy and Rolls, 2009; Herman and Polivy, 2008). With respect to energy expenditure, the design of neighborhoods and communities (i.e., the “built environment”) may influence an individual’s physical activity level (Saelens and Handy, 2008). Socioeconomic status and cognitive factors also play important roles in energy balance behaviors, such as an individual’s perceived control over diet and physical activity behaviors and social norms (Kremers et al., 2005; Stringhini et al., 2010). TABLE 22-2 Much current research is focused on understanding the relative contribution of the modern environment to the growing prevalence of obesity in both children and adults (Cohen, 2008). Among the factors under investigation are the impacts of portion size and food advertising on food selection and intake. Short-term food and energy intake is increased when there are many palatable foods from which to choose and when the food is served in large portions (Wansink, 2004). Likewise, aggressive advertising of high-calorie foods and increased participation in sedentary behaviors have been highlighted as environmental pressures that promote excessive eating, reduced energy expenditure, and, consequently, increased risk for excessive weight gain (Peters et al., 2002). Research is also directed at investigating interrelationships between biology and energy balance behaviors. For example, the association of food intake and food reward (Neary and Batterham, 2010), decision making about food intake in an environment where many constraints on availability and many costs associated with obtaining food have been removed (Rowland et al., 2008), and sex differences in regulation of food intake induced by physical activity (Hagobian and Braun, 2010) are topics of recent investigations. The CNS (brain and spinal cord) orchestrates the control of energy balance via a complex homeostatic pathway that has three main parts. As illustrated in Figure 22-3, the central part of this system involves specific regions within the CNS that detect and integrate signals reflecting changes in energy availability and mediate appropriate modifications of energy intake and expenditure. Inputs or afferent signals that provide information about the periphery, including status of energy intake, energy expenditure, and available energy stores, feed into the CNS. Afferent signals can be neural or humoral. The final part involves the outputs or efferent signals from the brain that direct a coordinated response to alter energy intake and energy expenditure or change partitioning of nutrients to achieve energy homeostasis. These afferent and efferent signal pathways constitute the essential elements of a feedback scheme, conceptually similar to the functional elements of a common household heating system in which a temperature sensor is linked to a system that adjusts heat output to maintain a specific temperature. Although the regulation of energy balance is complex and involves many brain centers and neuronal circuits, the hypothalamus and brainstem (Figure 22-4) play critical roles in relaying afferent signals from the peripheral tissues as well as processing efferent signals that modulate food intake and energy expenditure.
Control of Energy Balance∗
Energy Balance
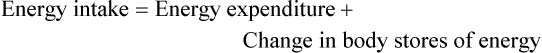
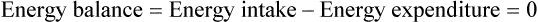
ENERGY FORM
STORAGE SITE
30-kg Child∗
70-kg Adult Man†
kg
kcal
kg
kcal
Triacylglycerol
Adipose tissue
4.5
42,000
15
140,000
Protein
Muscle
1.5
6,300
6
25,000
Glycogen
Liver and muscle
0.13
500
0.35
1,400
Glucose or lipid
Body fluids
0.011
40
0.025
100
Nutrient Balance
Relative Stability of Body Weight
Set Point Theory
The Settling Point Theory
Biological Bias Toward Body Weight Gain
Behavioral Aspects of Control of Energy Intake and Energy Expenditure
ENERGY INTAKE
ENERGY EXPENDITURE
Individual
Dietary macronutrient composition
Sedentary behaviors
Dietary energy density
Work-related activity
Food form (solid, liquid)
Activities of daily living
Palatability
Leisure time activity
Food variety and availability
Planned exercise
Food cost
Portion size
Sleep duration
Mental work
Environmental
Dining partners
Built environment
Food accessibility and variety
Family/social environment
Family and home environment
Biological Control of Energy Intake and Energy Expenditure
Regulation of Energy Homeostasis
Central Role of the Hypothalamus and Brainstem
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Control of Energy Balance