http://evolve.elsevier.com/Edmunds/NP/
Agents in this class can be divided into progestin-only agents and combination agents including both estrogen and progestin. Combined hormonal contraceptives are formulated for oral, transdermal, and intravaginal routes of administration. Progestin-only agents may be administered orally, intramuscularly, subcutaneously, in intrauterine systems, and via implants. Oral contraceptives (OCs) are commonly known as “birth control pills” or “the pill.”
This chapter is intended to provide the basic information necessary to understand contraception. Because these medications are used to prevent an unwanted pregnancy and not to treat a disease, the patient should always be invited to share in the decision making about contraceptive methods. This, like other components of medicine and surgery, makes selecting the best contraceptive method more of an art than a science. This is a rapidly changing field, with new information and new products. The provider will want to read further on this topic and check for the latest information before prescribing contraceptives.
The first OC pills, which were developed in the 1950s, contained only high doses of progestin. These early pills caused significant breakthrough bleeding (BTB). During manufacture, they became “contaminated” with estrogen. The women who took the contaminated pills had less BTB, so the estrogen was left in. The first combined birth control pill, which contained much higher doses of hormones than are found in the pills used today, was released in 1960.
In the 1970s, researchers first noted a dose-response relationship between high-estrogen pills and the risk of venous thromboembolism. This was caused by the action of oral estrogen on hepatic induction of fibrinogen, which increased the tendency to form blood clots. Early pills had roughly three times more estrogen and nine times more progestin than the pills that are available today. As doses of estrogen have been decreased to below 50 mcg of ethinyl estradiol (EE), thrombus risk has markedly diminished.
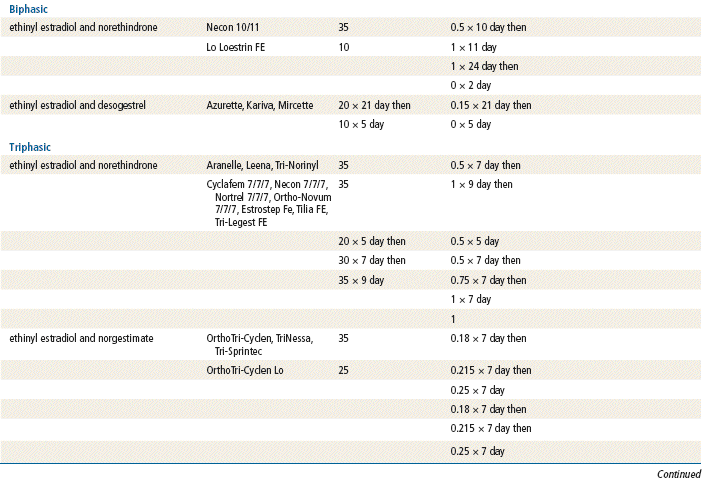
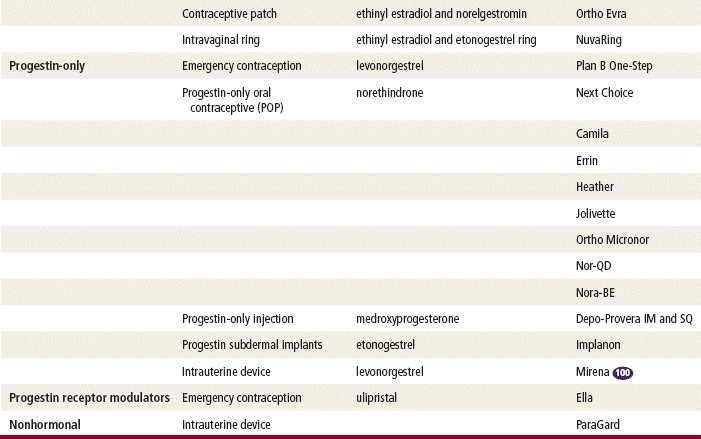
The original hormonal contraceptives were monophasic (same daily dose). Progestin-only pills and phasic preparations (estrogen and/or progestin doses that change during the 21-day cycle in an attempt to mimic the typical menstrual cycle) were introduced in the 1970s. Longer-acting progestin-only methods (e.g., medroxyprogesterone, a 3-month progestin-only injection; and Norplant, a 5-year implanted rod system) were approved for the U.S. market in the 1980s. Norplant was taken off the market because of adverse publicity over litigation regarding dose standardization and removal problems. Implanon, a single-rod implant, was introduced in 2006. A new radiopaque implant, Nexplanon, is now available.
In 2002, transdermal and vaginal hormone delivery systems were approved for use in preventing pregnancy. The trend for new contraceptives continues with the introduction of additional combination OCs with lower estrogen levels and different formulations of estrogen and progesterone. In 2003, extended-cycle contraceptives that contain levonorgestrel and an ethinyl estradiol tablet were introduced. Women who take this product experience fewer menstrual cycles per year, or menstruation may be suppressed entirely. Other new products include formulations with more active pills and shortened placebo intervals.
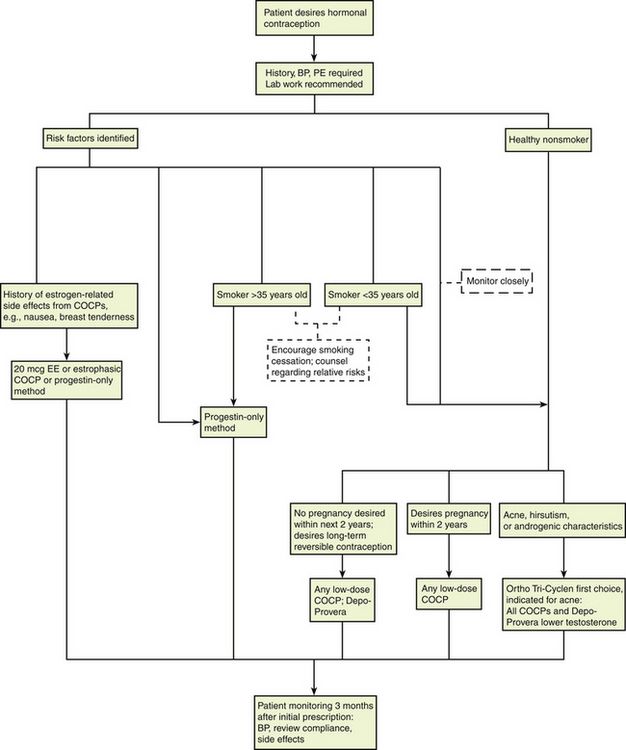
Therapeutic Overview
The Menstrual Cycle
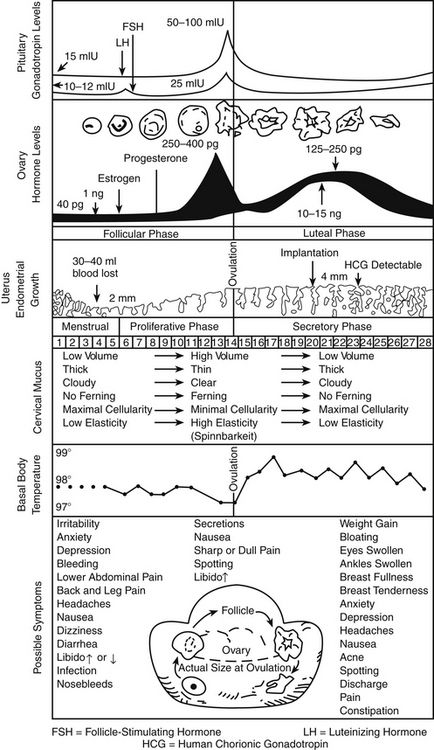
The menstrual cycle, which is a 28-day preparation cycle, is a classic negative feedback loop involving the hypothalamus, anterior pituitary gland, and ovaries. Menstruation begins on day 1 of the cycle, with shedding of the endometrium in response to declining estrogen and progesterone levels. Cycles consist of a follicular phase and a luteal phase that are nearly equal in length. Cycle lengths vary among individuals, and variability is increased in adolescents and in women who are close to perimenopause. Most variation occurs in the follicular phase. At the end of a menstrual cycle in which a pregnancy has not occurred, the hypothalamus responds to low circulating levels of estrogen and progesterone and secretes gonadotropin-releasing hormone (GnRH). GnRH stimulates the anterior pituitary gland to secrete follicle-stimulating hormone (FSH) and luteinizing hormone (LH); these stimulate the ovaries to begin the ovulation cycle anew. The ovaries are the female gonads, which are responsible for secretion of the sex steroid hormones estrogen, progesterone, and testosterone. These gonads also store the ovarian follicles, which in turn are the home of oocytes. When a woman is born, her ovaries contain approximately 2 million follicles, and by puberty, 300,000 remain to carry her to menopause. Only about 500 follicles ovulate during the reproductive years.
FSH stimulates follicular growth and ovarian development. During each cycle, 18 to 20 follicles are stimulated; all except one dominant follicle eventually die off. The dominant follicle produces estrogen and promotes an environment favorable to healthy development of the follicle and its oocyte, in preparation for eventual ovulation. Enlargement of this dominant follicle causes estrogen levels to gradually increase. Near the middle of a cycle (around day 12 to 13 of a 28-day cycle), estrogen in the blood reaches a critical level that sends a hormonal message to the pituitary gland indicating that the follicle is mature and that ovulation is imminent. The pituitary gland responds by sending out the hormonal messenger, LH. The midcycle LH surge induces ovulation. The follicle, now a “bubble” on the side of the ovary, bursts and releases the egg into the ampulla of the fallopian tube. At the site of ovulation, the remains of the follicle form a cyst, called the corpus luteum. At this time, estrogen levels drop sharply and then stabilize as the corpus luteum assumes the role of hormone production, with production of progesterone predominating over that of estrogen.
Progesterone levels are very low during the first half of the cycle but climb sharply after ovulation, with the appearance of the corpus luteum. Progesterone dominates in the second half of the cycle, or the luteal phase. The corpus luteum cyst, maintained by LH, is a “hormone factory” that produces large amounts of progesterone. The corpus luteum typically remains functional for approximately 12 days. If no additional hormonal message (e.g., fertilization and implantation) is sent, the corpus luteum dies and regresses on day 26 of a 28-day cycle. With the demise of the corpus luteum, blood progesterone levels drop dramatically. By the last day of the cycle, baseline progesterone and estrogen levels signal the hypothalamus to start a new menstrual cycle.
Endometrial Changes
The thickness of the endometrial, or uterine, lining parallels the changing hormone levels, shedding from day 1 to about day 5 back to almost basement lining. Increasing estrogen levels in the first half of the cycle stimulate endometrial proliferation. Progesterone, which is produced in large amounts by the corpus luteum in the second half of the cycle, stabilizes the endometrium by increasing its blood supply and glycogen stores, producing a secretory endometrium that is receptive to implantation. Under the influence of progesterone, the endometrium ceases to grow in thickness but becomes significantly denser. The proliferative effects of estrogen must be present in the first half of the cycle for progesterone to produce a secretory endometrium. Without the stabilizing influence of progesterone, the endometrium would continue to thicken but, if unsupported by an adequate blood supply, would shed irregularly. This condition is seen in the woman with anovulatory cycles, who, lacking the secretion of progesterone by the corpus luteum has unpredictable, often heavy noncyclic bleeding as a result of lack of production of progesterone. Similarly, the secretion of large amounts of progesterone without adequate secretion of estrogen (i.e., unopposed progesterone) produces a thin endometrium that is not receptive to implantation.
If fertilization occurs and the egg implants, the embryo and the developing placenta produce human chorionic gonadotropin (hCG), which maintains the corpus luteum cyst for about 100 days until the placenta is advanced enough to produce its own progesterone. After the placenta matures, the corpus luteum declines.
In summary, the uterine lining proliferates in response to rising estrogen levels in the first half of the cycle. In the second half of the cycle, progesterone maintains a secretory endometrium. At the end of the menstrual cycle during which conception did not take place, progesterone and estrogen levels drop off, and the unsupported uterine lining sheds (Figure 54-1).
Hormone Physiology
Hormones are chemical messengers produced in the body that travel through the bloodstream from a gland to a distant site, where they exert their effects on specific organs or tissue.
The ovary produces three classes of sex steroid hormones: progestins, androgens, and estrogens. These steroid hormones can be synthesized in the ovaries in situ or derived from serum cholesterol that enters the ovaries. Several steps are included in the steroid biosynthesis pathway. Through a series of intermediary steps, cholesterol (a 27-carbon molecule) is broken down to progestins (21-carbon molecules), then to androgens (19-carbon molecules), and finally to estrogens (18-carbon molecules). During steroidogenesis, the number of carbon atoms can be reduced but never increased. Therefore, the metabolic breakdown of progestins can have progestational, androgenic, and estrogenic actions. The metabolic breakdown of androgens can have androgenic and estrogenic actions, but the metabolic breakdown of estrogens will have only estrogenic actions. In women, the principal circulating sex hormones—estrogen, estradiol, and the androgen testosterone—are also directly produced by the ovary. Most estradiol and testosterone (69%) are bound to sex hormone–binding globulin (SHBG), a protein carrier. Roughly 30% is loosely bound to albumin, leaving only 1% unbound and free. It is this free 1% that determines the biologic effects of estradiol and testosterone. Estrogen administration increases SHBG levels, thereby decreasing the quantities of free, or active, sex steroids. Progestins and androgens decrease SHBG, thereby increasing the quantities of free sex steroids. All combined oral contraceptive pills (COCPs) increase SHBG, although some do so more than others, depending on the progestin component and the ratio of estrogen to progestin. Because all COCPs increase SHBG, free testosterone is always decreased by some degree in women who are taking COCPs.
Progestins: Progesterone is secreted in significant amounts (20 to 30 mg/day) in the second half of the normal menstrual cycle by the corpus luteum. During the first half of the cycle, estrogen dominates and progesterone is secreted in minute amounts (2 to 3 mg/day) by the ovaries and the adrenals. During pregnancy, the placenta secretes very large amounts of progesterone.
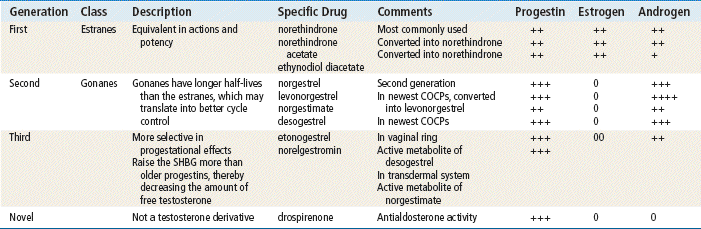
The plasma half-life of progesterone is only 5 to 10 minutes, after which it is degraded to other steroids that have no progestational effect. Progesterone in its natural state cannot be used in oral form because of its rapid breakdown by the liver, so chemical modifications of synthetic progestins in hormonal contraceptives were made to deliberately slow down liver metabolism, making it possible to use the oral route (Table 54-1).
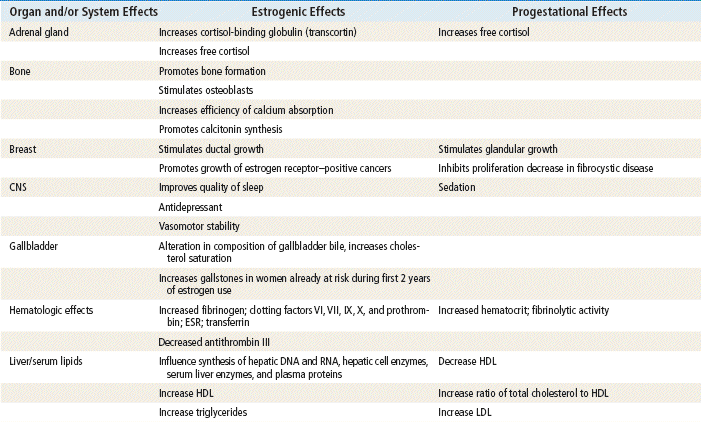
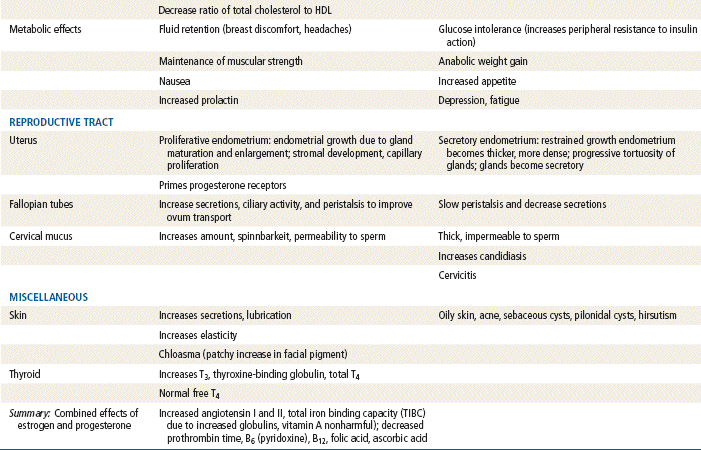
Effects of progesterone in the body are limited and are seen primarily in the reproductive tract and the breast; other effects are evident as changes in metabolism. Progesterone is the dominant hormone of pregnancy; it produces a secretory endometrium, decreases uterine contractions, and stimulates alveolar epithelial growth in the breast.
Norethindrone, the progestin originally used in OCs, was derived from ethisterone, an orally active form of testosterone. The removal of a 19-carbon molecule from ethisterone results in the formation of norethindrone and changes the major effect from that of an androgen to that of progesterone, but the androgenic component is never totally eliminated, and the potential for anabolic and androgenic effects remains. See Table 54-2 for biologic effects of progestins and estrogen. This is a dose–effect relationship: The lower the dose of progestins given, the lesser is the androgenic effect. At today’s very low doses of progestin in COCPs, clinical effects are usually negligible. As with estrogens, serious side effects, especially adverse serum lipid changes, have been associated with high doses of progestins; therefore, the lowest effective doses available should be used. Through the years, changes have been made in the chemical structure of progestins in the quest to produce new progestins that have more potent progestational activity with fewer androgenic side effects. Many different progestins are marketed in the United States for use in hormonal contraception.
Estrogens: The human body produces three estrogens: estradiol, estrone, and estriol. The major estrogen is estradiol. It is 12 times more potent than estrone and 80 times more potent than estriol. The primary source of estrogen in women with normal menstrual cycles is estradiol, which is secreted by the ovary. The ovarian follicle secretes 100 to 300 mcg of estradiol daily, depending on the phase of the menstrual cycle. Estradiol levels of greater than 200 mcg are required for ovulation to occur. The ovary also secretes small amounts of estrone, but most comes from adrenal androgens that are converted to estrone in peripheral tissues, such as adipose, skin, and muscle. Estriol is a metabolite of estrone and estradiol.
Estrogens are conjugated in the liver to form glucuronides and sulfates. About one fifth of these conjugated products are excreted in the bile; the rest are excreted by the kidneys. Synthetic estrogens, such as ethinyl estradiol (EE), are degraded very slowly in the liver and other tissues, which causes their high intrinsic potency.
Estrogens are important in the development and maintenance of the female reproductive tract. Estrogen has effects throughout the body that are most notable in the breasts, bones, liver, and urogenital structures. See Table 54-1 for estrogen effects on the body.
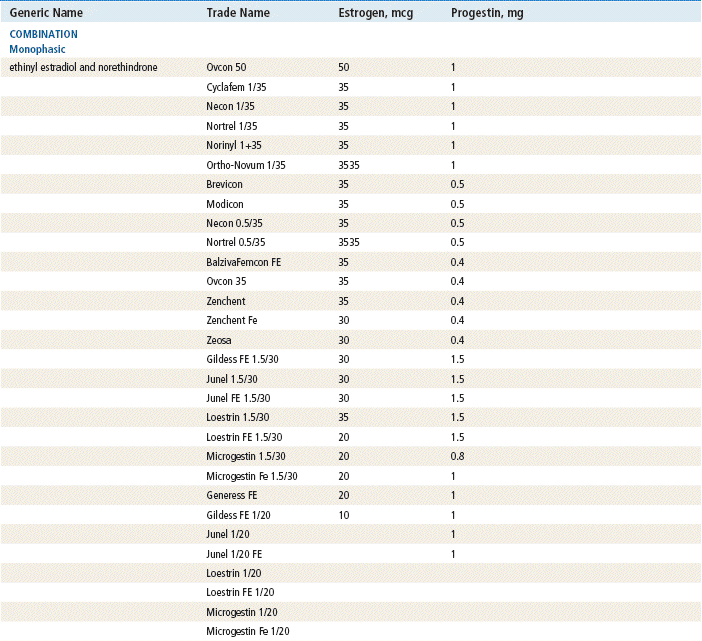
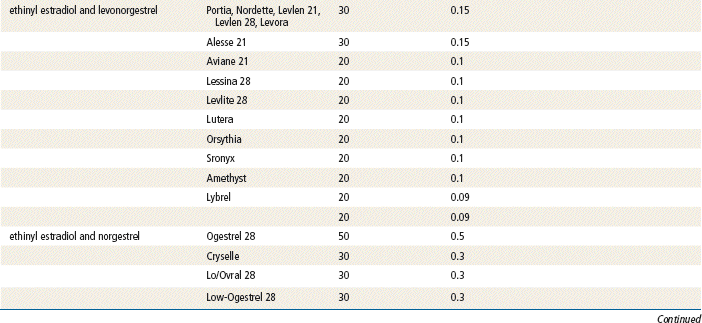
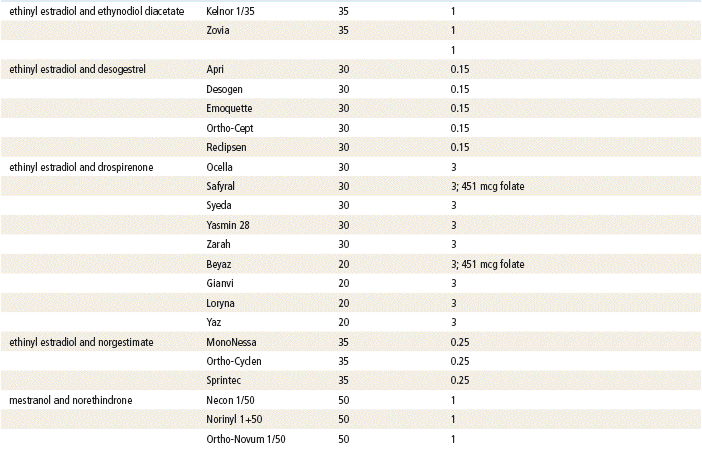
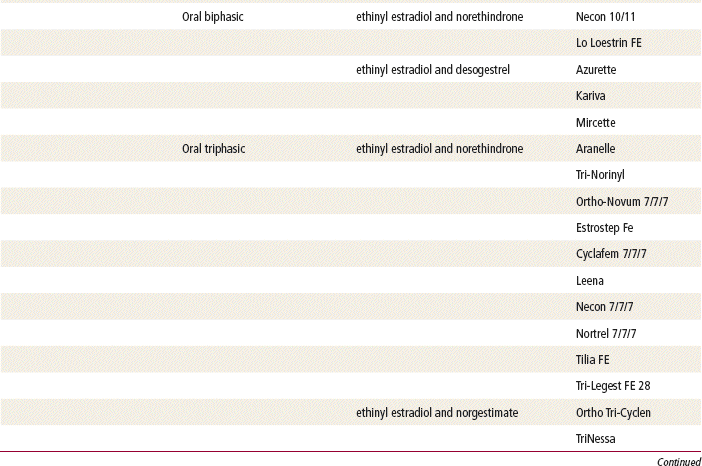
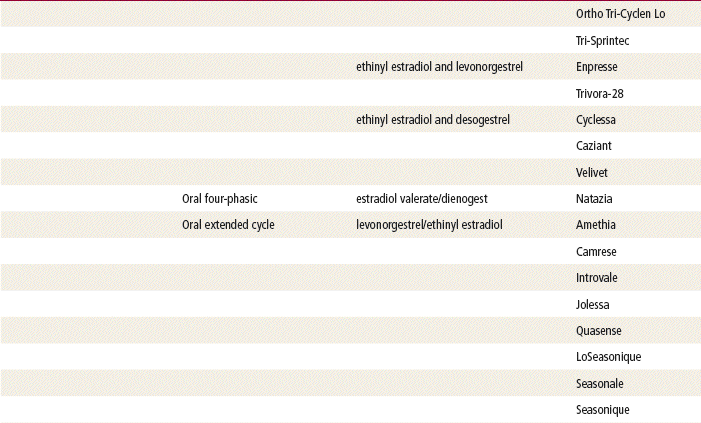
Two estrogenic compounds are used in COCPs in the United States: EE and mestranol. Mestranol is considered pharmacologically weaker because it must first be converted to EE. Therefore, unconjugated EE is the active estrogen in the blood in both mestranol and EE. All of the low-dose pills are low-estrogen pills. They contain ≤35 mcg of estrogen pills (EE). Mestranol is available in only a few pills in a 50-mcg dose, which is roughly equivalent to 35 mcg EE. A relatively safe dose of EE is ≤35 mcg. Pills that include 50-mcg EE are reserved for women on medications that induce liver enzymes that degrade estrogen, requiring higher initial doses, and they are rarely used. It is more common practice to use other methods with women who require higher-dose pills. High-dose EE should be given under special circumstances and generally is not used in primary care. In this chapter, COCPs are assumed to contain ≤35 mcg of EE (Table 54-3).
Mechanism of Action
Hormonal contraceptive agents interfere with the hypothalamic-pituitary-ovarian negative feedback loop to inhibit ovulation. Constant, low levels of estrogen and/or progestin have suppressive effects at both the hypothalamus and the pituitary, inhibiting GnRH, FSH, and LH. Suppression of FSH and LH inhibits ovulation. Additional contraceptive effects stem from actions on the cervical mucus (thickening, to prevent passage of sperm) and the endometrium (providing a lining that is hostile to implantation). Hormonal contraceptives are not abortifacients. An implanted pregnancy will not be disrupted by their administration.
The primary contraceptive action of progestin in hormonal contraceptives is suppression of the LH surge, which inhibits ovulation. When given in supraphysiologic doses, progestins produce a decidualized endometrial bed with atrophied glands that is not receptive to implantation, as well as thick cervical mucus, which hampers sperm transport and may also impair ovum transport by slowing peristalsis and decreasing secretions in the fallopian tubes.
The contraceptive action of estrogens in hormonal contraceptives is primarily the result of suppression of FSH. Without FSH, no dominant follicle emerges and ovulation is inhibited. Estrogen contributes to endometrial stability and avoidance of irregular shedding and/or bleeding. Estrogen increases intracellular progesterone receptors, making the given dose of progestin more effective.
Emergency contraceptives (ECs) are thought to interfere with ovulation. Other possible actions include (1) disruption of the endometrium to inhibit implantation and (2) alteration in tubal transport of sperm or ova to prevent fertilization. ECs are not effective with an existing pregnancy.
Treatment Principles
Evidence-Based Recommendations
No evidence-based medicine studies regarding use of these medications for contraception have been reported. Studies have described the relative merits of these drugs for patients with dysmenorrhea, PMS, and menorrhagia.
Cardinal Points of Treatment
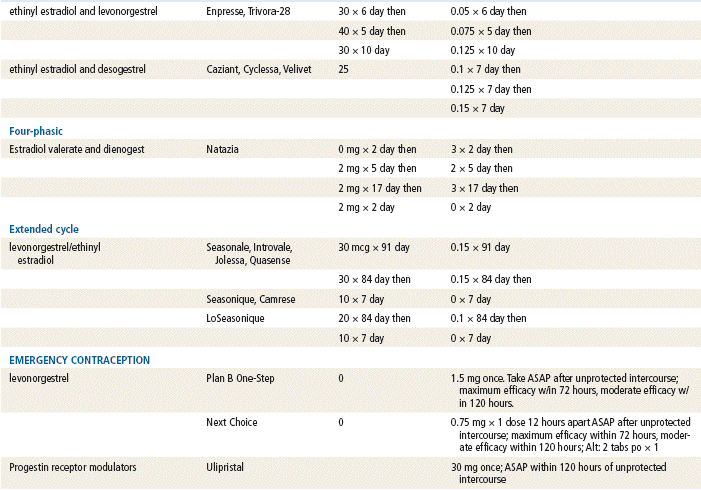
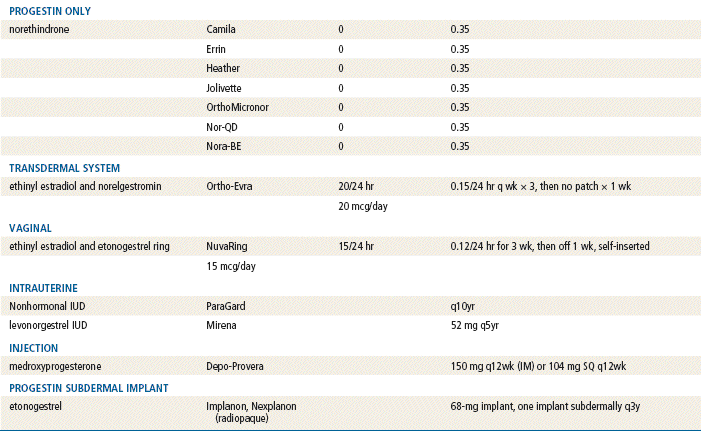
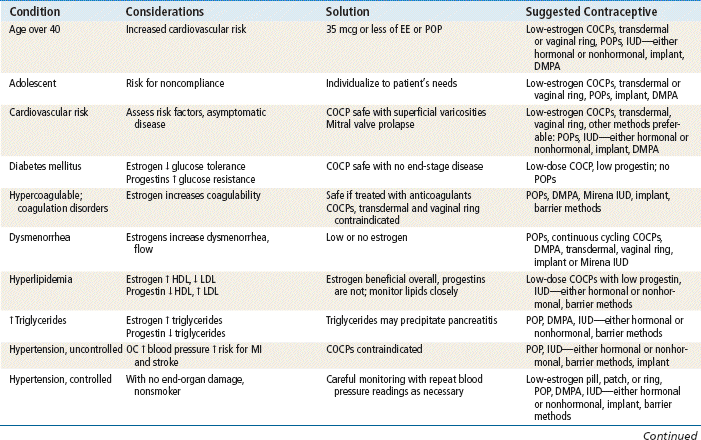
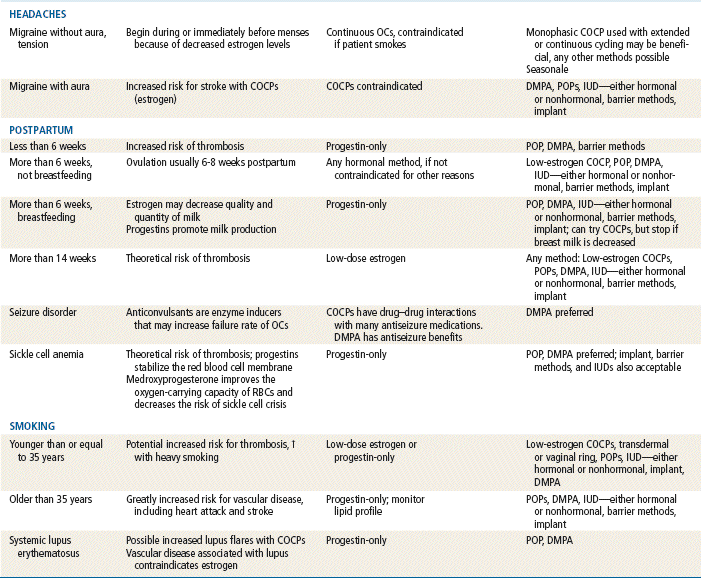
TABLE 54-4
Comparison of Hormonal Methods
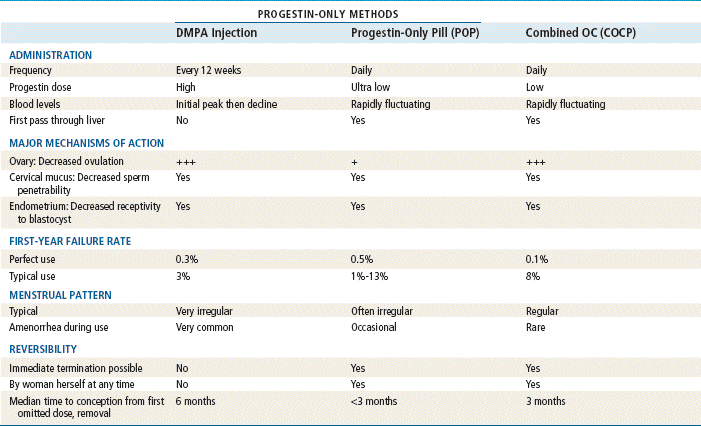
Modified from Hatcher RA: Contraceptive technology, ed 18 rev, New York, 2007, Ardent Media Trade, Inc.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




 Top 100 drug.
Top 100 drug.