4 CONNECTIVE TISSUE
Classification
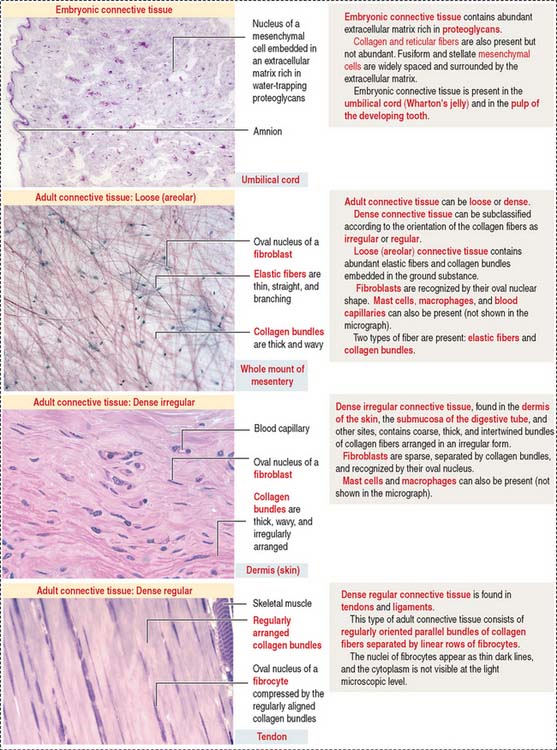
Connective tissue can be classified into three major groups (Figure 4-1): embryonic connective tissue, adult connective tissue, and special connective tissue.
In addition, reticular and elastic fibers predominate in irregular connective tissue.
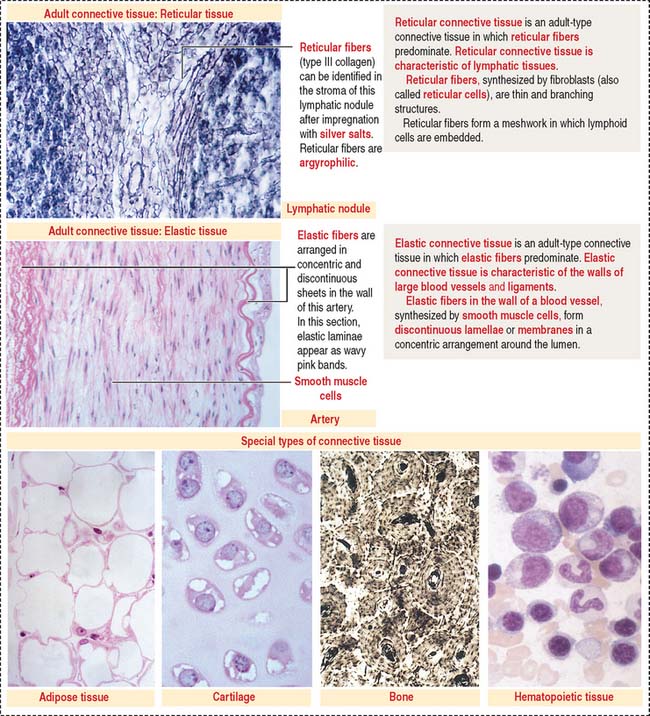
The special connective tissue comprises types of connective tissue with special properties not observed in the embryonic or adult connective tissue proper. There are four types of special connective tissue (Figure 4-2):
Adipose tissue has more cells (called adipose cells or adipocytes) than collagen fibers and ground substance. This type of connective tissue is the most significant energy storage site of the body.
The hematopoietic tissue is found in the marrow of selected bones. This type of connective tissue is discussed in Chapter 6, Blood and Hematopoiesis.
Cartilage and bone are also regarded as special connective tissue but are traditionally placed in separate categories. Essentially, cartilage and bone are dense connective tissues with specialized cells and ground substance. An important difference is that cartilage has a noncalcified ECM, whereas the ECM of bone is calcified. These two types of specialized connective tissue fulfill weight-bearing and mechanical functions that are discussed later (see Cartilage and Bone).
Cell components of connective tissue
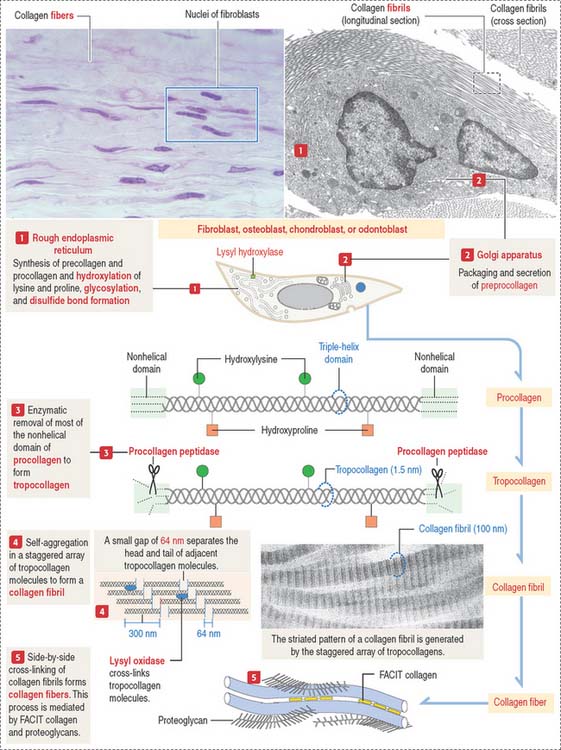
The fibroblast synthesizes and continuously secretes mature proteoglycans and glycoproteins and the precursor molecules of various types of collagens and elastin. Different types of collagen proteins and proteoglycans can be recognized as components of the basement membrane. As you may remember, type IV collagen is found in the basal lamina and type III collagen appears in the reticular lamina as a component of reticular fibers (see Boxes 4-A and 4-B). Heparan sulfate proteoglycans and the glycoprotein fibronectin are two additional products of the fibroblast that appear in the basement membrane. The protein collagen is a component of collagen and reticular fibers. However, elastic fibers do not contain collagen.
Box 4-A Distribution of collagen
Observed in hyaline and elastic cartilage as fibrils thinner than type I collagen.
Box 4-B Cell types making collagen
Collagen: Synthesis, secretion, and assembly
Collagens are generally divided into two categories: fibrillar collagens (forming fibrils with a characteristic banded pattern), and nonfibrillar collagens (see Box 4-C).
Box 4-C Characteristics of collagens
The synthesis of collagen starts in the rough endoplasmic reticulum (RER) following the typical pathway of synthesis for export from the cell (Figure 4-3).
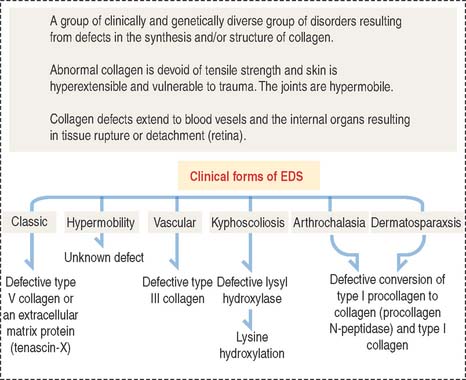
Clinical significance: Ehlers-Danlos syndrome
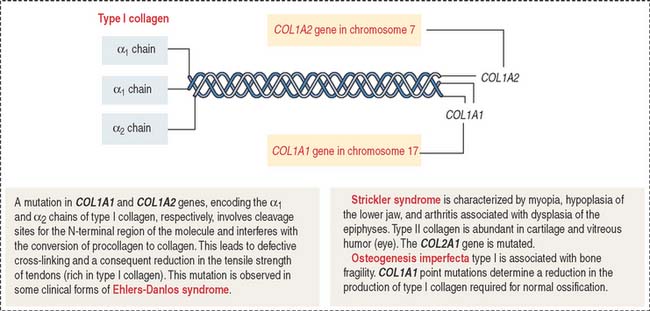
Ehlers-Danlos syndrome is clinically characterized by hyperelasticity of the skin (Figure 4-4) and hypermobility of the joints. The major defect resides in the synthesis, processing, and assembly of collagen. Several clinical subtypes are observed. They are classified by the degree of severity and the mutations in the collagen genes. For example, the vascular type form of Ehlers-Danlos syndrome—caused by a mutation in the COL3A1 gene—is associated with severe vascular alterations leading to the development of varicose veins and spontaneous rupture of major arteries. A deficiency in the synthesis of type III collagen, prevalent in the walls of blood vessels, is the major defect. Arthrochalasia and dermatosparaxsis types of Ehlers-Danlos syndrome display congenital dislocation of the hips and marked joint hypermobility. Mutations in the COL1A1 and COL1A2 genes (Figure 4-5), encoding type I collagen, and procollagen N-peptidase gene disrupt the cleavage site at the N-terminal of the molecule and affect the conversion of procollagen to collagen in some individuals.
Elastic fibers: Synthesis, secretion, and assembly
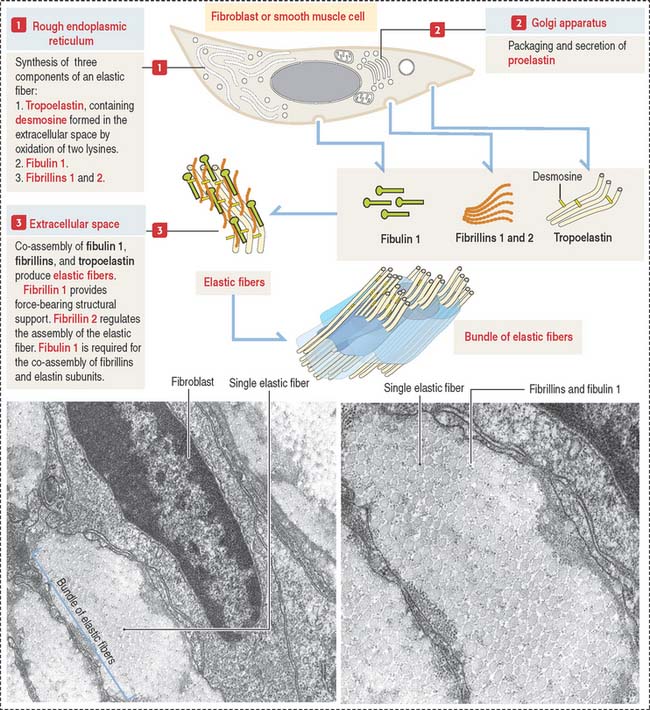
Like collagen, the synthesis of elastic fibers involves both the RER and the Golgi apparatus (Figure 4-6).
Elastic fibers are synthesized by the fibroblast (in skin and tendons), the chondroblast, the chondrocyte (in elastic cartilage of the auricle of the ear, epiglottis, larynx, and auditory tubes), and smooth muscle cells (in large blood vessels like the aorta and in the respiratory tree).
Proelastin, the precursor of elastin, is cleaved and secreted as tropoelastin. In the extracellular space, tropoelastin interacts with fibrillins and fibulin 1 to organize elastic fibers, which aggregate to form bundles of elastic fibers. Tropoelastin contains a characteristic but uncommon amino acid: desmosine. Two lysine residues of tropoelastin are oxidized by lysyl oxidase to form a desmosine ring that cross-links two tropoelastin molecules. Cross-linking enables the stretching and recoil of tropoelastin, like rubber bands. Elastic fibers do not contain collagen. Elastic fibers are produced during embryonic development and in adolescence but not so much in adults. Although elastic fibers are resilient during human life, many tissues decrease elasticity with age, in particular the skin, which develops wrinkles.
Clinical significance: Marfan syndrome
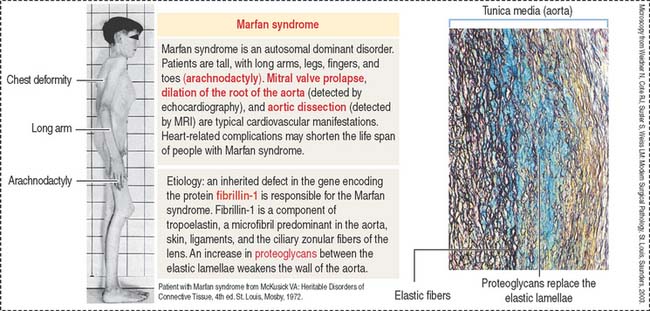
Marfan syndrome is an autosomal dominant disorder in which the elastic tissue is weakened. Defects are predominantly observed in three systems: the ocular, skeletal, and cardiovascular systems. The ocular defects include myopia and detached lens (ectopia lentis). The skeletal defects (Figure 4-7) include long and thin arms and legs (dolichostenomelia), hollow chest (pectus excavatum), scoliosis, and elongated fingers (arachnodactyly).
Defects observed in Marfan syndrome are caused by poor recoiling of the elastic lamellae dissociated by an increase in proteoglycans (see Figure 4-7). In the skeletal system, the periosteum, a relatively rigid layer covering the bone, is abnormally elastic and does not provide an oppositional force during bone development, resulting in skeletal defects.
A mutation of the fibrillin 1 gene on chromosome 15 is responsible for Marfan syndrome. Fibrillin is present in the aorta, suspensory ligaments of the lens (see Chapter 9, Sensory Organs: Vision and Hearing), and the periosteum (see Bone). A homologous fibrillin 2 gene is present on chromosome 5. Mutations in the fibrillin 2 gene cause a disease called congenital contractural arachnodactyly. This disease affects the skeletal system, but ocular and cardiovascular defects are not observed.
Macrophages
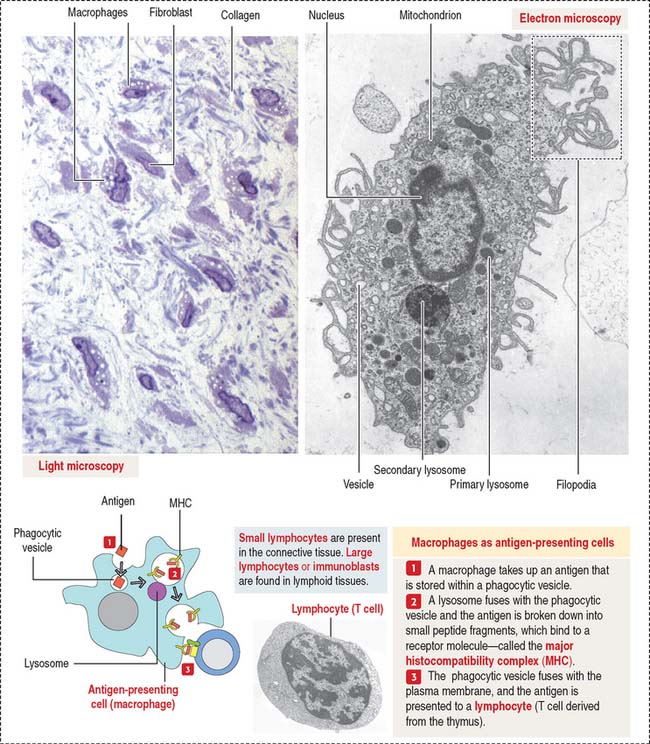
Macrophages have phagocytic properties and derive from monocytes, cells formed in the bone marrow (Figure 4-8).
Monocytes circulate in blood and migrate into the connective tissue, where they differentiate into macrophages. Macrophages have specific names in certain organs; for example, they are called Kupffer cells in the liver, osteoclasts in bone, and microglial cells in the central nervous system. Macrophages migrate to the site of inflammation, attracted by certain mediators, particularly C5a (a member of the complement cascade; see Chapter 10, Immune-Lymphatic System). Macrophages in the connective tissue have the following structural features:
Macrophages of the connective tissue have three major functions:
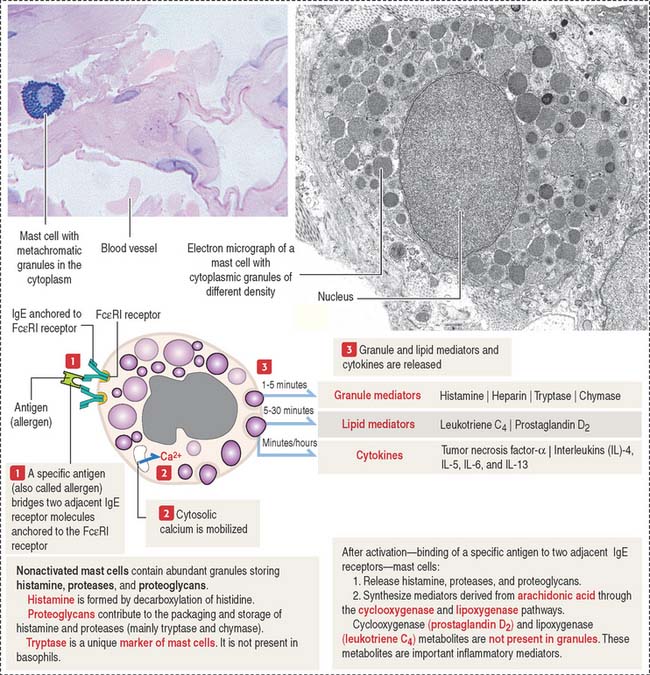
Mast cells
The mast cell is the source of vasoactive mediators contained in cytoplasmic granules (Figure 4-9). These granules contain histamine, heparin, and chemotactic mediators to attract monocytes, neutrophils, and eosinophils circulating in blood to the site of mast cell activation.
Connective tissue mast cells differ from mucosal mast cells in the number and size of metachromatic (see Box 4-D) cytoplasmic granules, which tend to be more abundant in connective tissue mast cells. Although these two cell populations have the same cell precursor, the definitive structural and functional characteristics of mast cells depend on the site of differentiation (mucosa or connective tissue).
Box 4-D Metachromasia
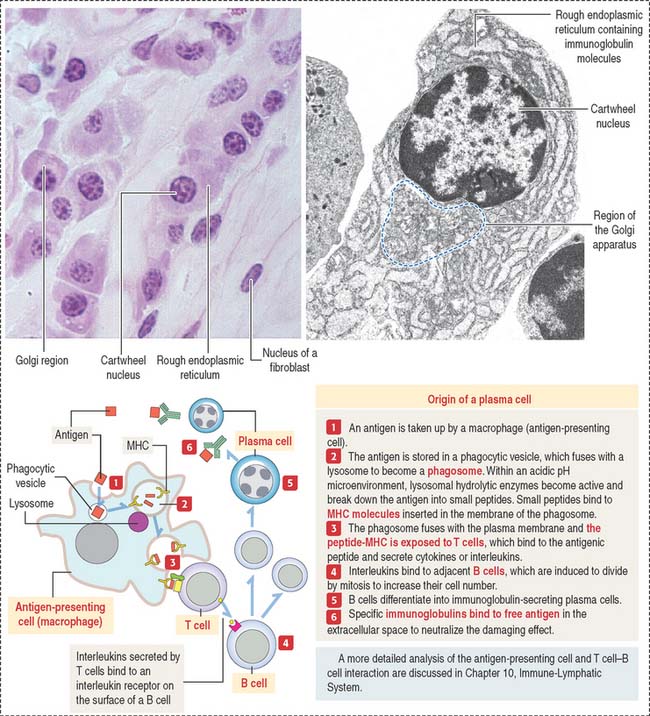
Plasma cells
The plasma cell, which derives from the differentiation of B lymphocytes (also called B cells), synthesizes and secretes a single class of immunoglobulin (Figure 4-10). We discuss in Chapter 10, Immune-Lymphatic System, details of the origin of plasma cells.
At the light microscopic level, most of the cytoplasm of a plasma cell is basophilic because of the large amount of ribosomes associated with the endoplasmic reticulum. A clear area near the nucleus is slightly acidophilic and represents the Golgi apparatus. The nucleus has a characteristic cartwheel configuration created by the particular distribution of heterochromatin.
Extracellular matrix
Recall that the basement membrane contains several ECM components such as laminin, fibronectin, various types of collagen, and heparan sulfate proteoglycan. In addition, epithelial and nonepithelial cells have receptors for ECM constituents. An example is the family of integrins with binding affinity for laminin and fibronectin. Integrins interact with the cytoskeleton, strengthening cell interactions with the ECM by establishing focal contacts or modifying cell shape or adhesion.
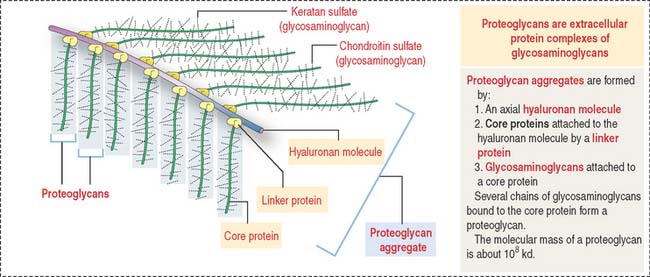
Proteoglycan aggregates (Figure 4-11) are the major components of the ECM. Each proteoglycan consists of glycosaminoglycans (GAGs), proteins complexed with polysaccharides. GAGs are linear polymers of disaccharides with sulfate residues. GAGs control the biological functions of proteoglycans by establishing links with cell surface components, growth factors, and other ECM constituents.
Degradation of the extracellular matrix
The expression of matrix metalloproteinase genes is regulated by cytokines, growth factors, and cell contact with the ECM.
Members of the family of matrix metalloproteinases include: