7 MUSCLE TISSUE
Muscle is one of the four basic tissues. There are three types of muscle: skeletal, cardiac, and smooth. All three types are composed of elongated cells, called muscle cells, myofibers, or muscle fibers, specialized for contraction. In all three types of muscle, energy from the hydrolysis of adenosine triphosphate (ATP) is transformed into mechanical energy.
SKELETAL MUSCLE
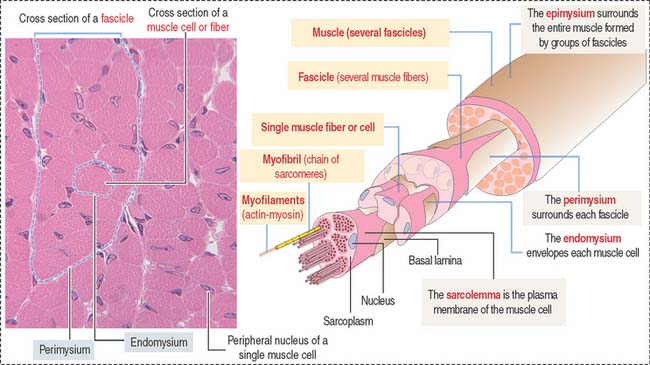
Muscle cells or fibers form a long multinucleated syncytium grouped in bundles surrounded by connective tissue sheaths and extending from the site of origin to their insertion (Figure 7-1). The epimysium is a dense connective tissue layer ensheathing the entire muscle. The perimysium derives from the epimysium and surrounds bundles or fascicles of muscle cells. The endomysium is a delicate layer of reticular fibers and extracellular matrix surrounding each muscle cell. Blood vessels and nerves use these connective tissue sheaths to reach the interior of the muscle. An extensive capillary network, flexible to adjust to contraction-relaxation changes, invests individual skeletal muscle cell.
Characteristics of the skeletal muscle cell or fiber
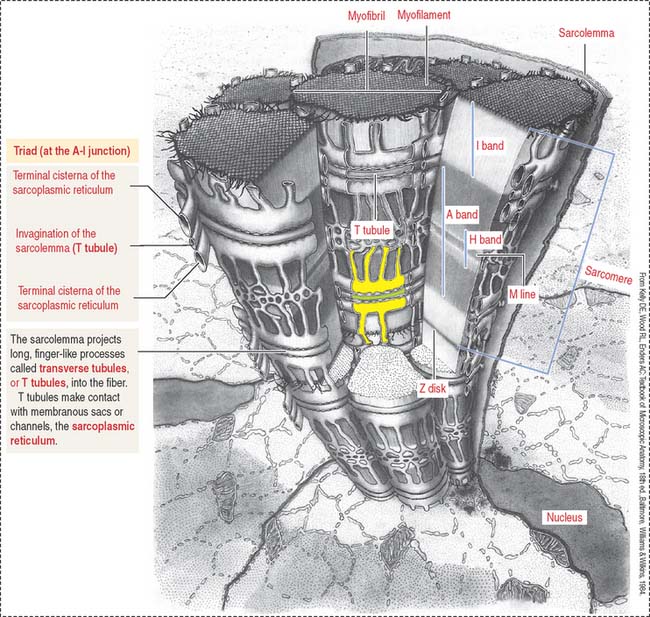
The plasma membrane (called the sarcolemma) of the muscle cell is surrounded by a basal lamina and satellite cells (Figure 7-2). We discuss the significance of satellite cells in muscle regeneration. The sarcolemma projects long, finger-like processes—called transverse tubules or T tubules—into the cytoplasm of the cell—the sarcoplasm. T tubules make contact with membranous sacs or channels, the sarcoplasmic reticulum. The sarcoplasmic reticulum contains high concentrations of Ca2+. The site of contact of the T tubule with the sarcoplasmic reticulum cisternae is called a triad because it consists of two lateral sacs of the sarcoplasmic reticulum and a central T tubule.
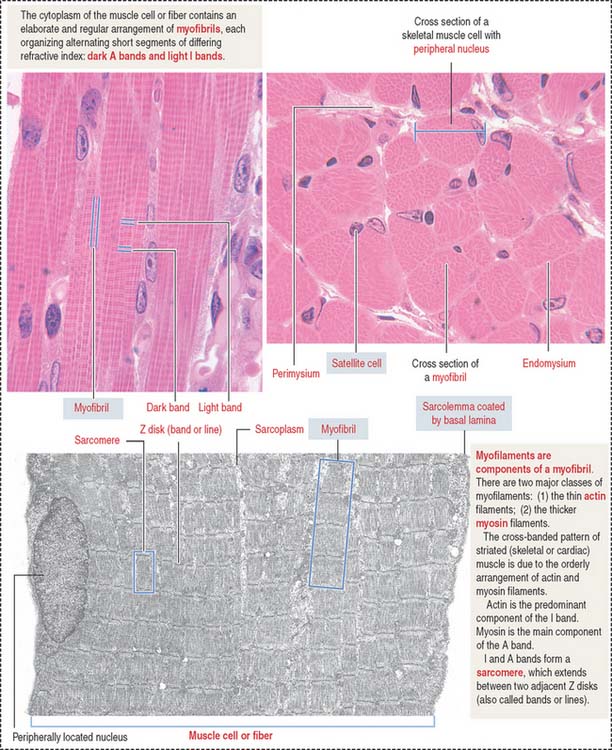
About 80% of the sarcoplasm is occupied by myofibrils surrounded by mitochondria (called sarcosomes). Myofibrils are composed of two major filaments formed by contractile proteins: thin filaments contain actin, and thick filaments are composed of myosin (see Figure 7-2).
The myofibril: A repeat of sarcomere units
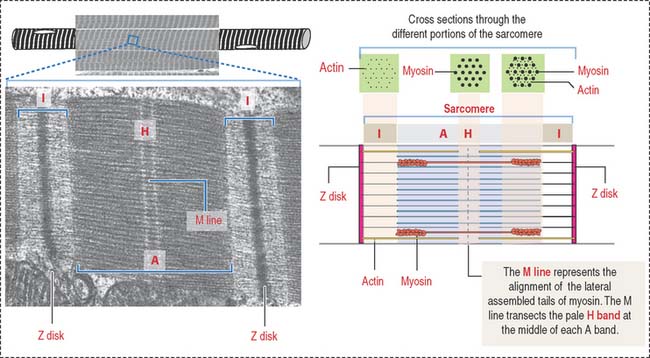
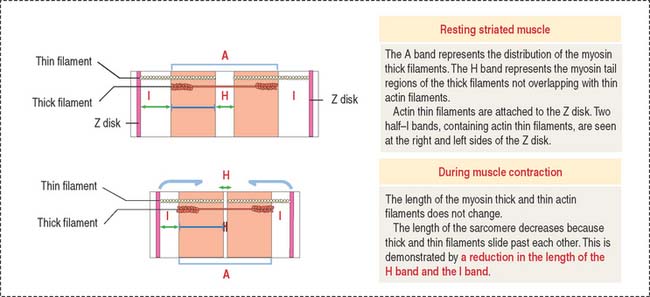
The sarcomere is the basic contractile unit of striated muscle (Figure 7-3). Sarcomere repeats are represented by myofibrils in the sarcoplasm of skeletal and cardiac muscle cells.
The arrangement of thick (myosin) and thin (actin) myofilaments of the sarcomere is largely responsible for the banding pattern observed under light and electron microscopy (see Figures 7-2 and 7-3). Actin and myosin interact and generate the contraction force. The Z disk forms a transverse sarcomeric scaffold to ensure the efficient transmission of the generated force.
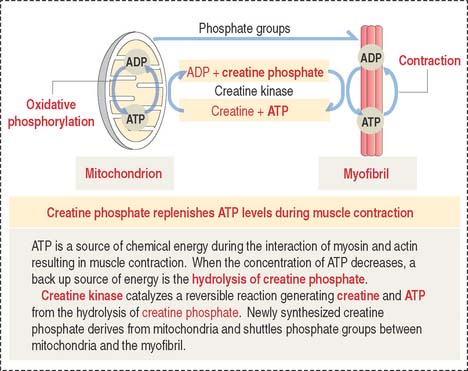
The A band is bisected by a light region called the H band (see Figures 7-3; 7-4). The major component of the H band is the enzyme creatine kinase, which catalyzes the formation of ATP from creatine phosphate and adenosine diphosphate (ADP). We discuss later how creatine phosphate maintains steady levels of ATP during prolonged muscle contraction.
Components of the thin and thick filaments of the sarcomere
F-actin, the thin filament of the sarcomere, is double-stranded and twisted. F-actin is composed of globular monomers (G-actin; see Cytoskeleton in Chapter 1, Epithelium). G-actin monomers bind to each other in a head-to-tail fashion, giving the filament polarity, with barbed (plus) and pointed (minus) ends. The barbed end of actin filaments inserts into the Z disk.
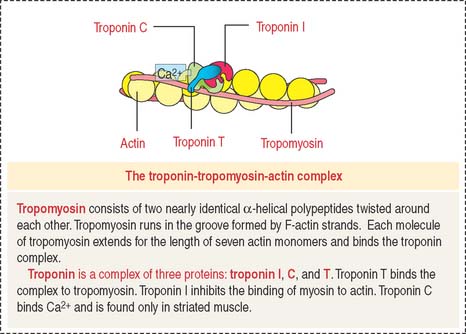
Tropomyosin consists of two nearly identical α-helical polypeptides twisted around each other. Tropomyosin runs in the groove formed by F-actin strands. Each molecule of tropomyosin extends for the length of seven actin monomers and binds the troponin complex (Figure 7-5).
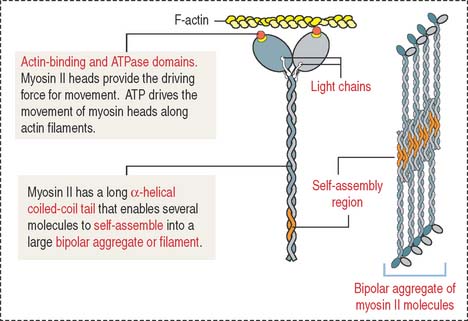
Myosin II consists of two identical heavy chains and two pairs of light chains (Figure 7-6; see Cytoskeleton in Chapter 1, Epithelium). At one end, each heavy chain forms a globular head. Two different light chains are bound to each head: the essential light chain and the regulatory light chain. The globular head has three distinct regions: (1) an actin-binding region; (2) an ATP-binding region; and (3) a light chain–binding region. Myosin II, like the other molecular motors kinesins and dyneins, use the chemical energy of ATP to drive conformational changes that generate motile force. As you recall, kinesins and dyneins move along microtubules. Myosins move along actin filaments to drive muscle contraction.
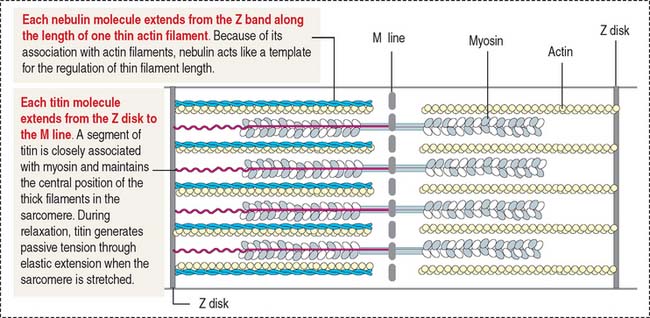
Nebulin (Figure 7-7) is associated with thin (actin) filaments; it inserts into the Z disk and acts as a template for determining the length of actin filaments.
Titin (see Figure 7-7) is a very large protein with a molecular mass in the range of millions. Each molecule associates with thick (myosin) myofilaments and inserts into the Z disk, extending to the bare zone of the myosin filaments, close to the M line. Titin controls the assembly of the myosin myofilament by acting as a template. Titin has a role in sarcomere elasticity by forming a spring-like connection between the end of the thick myofilament and the Z disk.
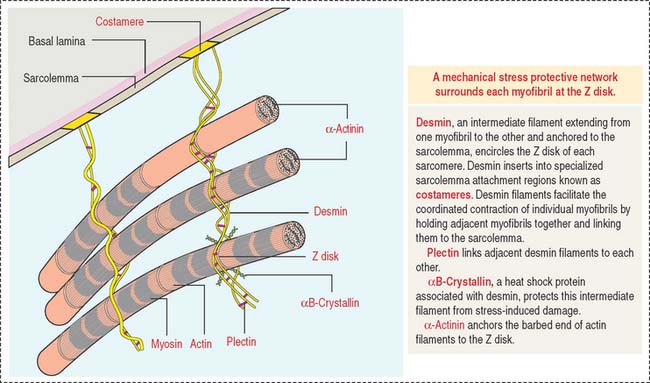
Desmin is a 55-kd protein that forms intermediate (10-nm) filaments. Desmin filaments encircle the Z disks of myofibrils and are linked to the Z disk and to each other by plectin filaments (Figure 7-8). Desmin filaments extend from the Z disk of one myofibril to the adjacent myofibril, forming a supportive latticework. Desmin filaments also extend from the sarcolemma to the nuclear envelope.
Mechanism of muscle contraction
During muscle contraction, the muscle shortens about one third of its original length. The relevant aspects of muscle shortening are summarized in Figure 7-9 as follows:
Creatine phosphate: A back up energy source
Figure 7-10 provides a summary of the mechanism of regeneration of creatine phosphate, which takes place in mitochondria and diffuses to the myofibrils, where it replenishes ATP during muscle contraction.
A depolarization signal travels inside the muscle by T tubules
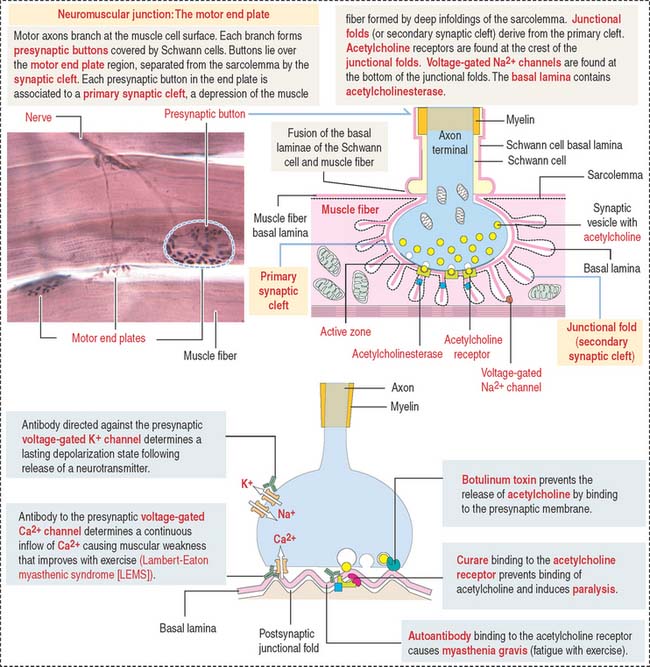
An excitation-contraction signal is generated by acetylcholine, a chemical transmitter released from a nerve terminal in response to an action potential. Acetylcholine diffuses into a narrow gap, called the neuromuscular junction, between the muscle and a nerve terminal (Figure 7-11). The action potential spreads from the sarcolemma to the T tubules, which transport the excitation signal to the interior of the muscle cell. Remember that T tubules form rings around every sarcomere of every myofibril at the A-I junction.
Box 7-A Functional types of muscle fibers
NEUROMUSCULAR JUNCTION: MOTOR PLATE
Synaptic buttons occupy a depression of the muscle fiber, called the primary synaptic cleft. In this region, the sarcolemma is thrown into deep junctional folds (secondary synaptic clefts). Acetylcholine receptors are located at the crests of the folds and voltage-gated Na+ channels are down into the folds (see Figure 7-11).