Beginning with % w/v, which is the most common concentration form, the active ingredient is usually a solid that is expressed in grams. In % v/v (usually seen with alcoholic dilutions), the active ingredient is a liquid and the amount used is expressed in milliliters. Finally, in % w/w, which is a solid mixed in a solid (such as what is observed in ointments), the active drug is again expressed in grams and the denominator, being also a solid, is expressed in grams as well.
Notice that for all concentration terms the denominator is based upon 100 parts (milliliters or grams) of final solution or mixture. So a 5% w/v refers to 5 g of active ingredient in 100 mL of final liquid preparation. One cannot determine how much diluent is needed since the amount of volume taken up by 5 g of active ingredient is not determinable.
5% v/v and w/w concentrations is another issue. In these cases, 5 mL of liquid active ingredient is contained in 100 mL of finished mixture. And 5 g of solid active ingredient is contained in 100 g of finished mixture. In both of these cases, since both the active ingredient and final mixture carry the same units, the amount of solute can be determined by subtraction. So 5 mL of liquid active ingredient is mixed with 95 mL of inert solute to make 100 mL of mixture. Likewise, 5 g of solid active ingredient is mixed with 95 g of inert solute to make 100 g of mixture.
Ratio strength is also used to designate concentration. Ratio strength designates the number of parts of total product that contain one part of active ingredient. It is useful to eliminate nonsignificant figures to the right of the decimal place.
Practice Exercises
Determine the concentration:
1. 325 g of active ingredient mixed with 400 g of inert powder
Answer
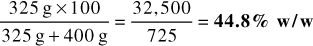
2. 230 mg of medication contained in 30 mL of mixture
Answer
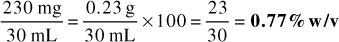
Or,
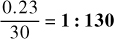
Answer
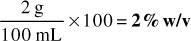
Answer
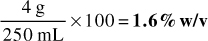
Answer
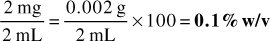
Answer
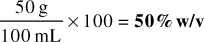
Answer
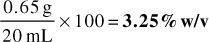
Answer
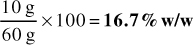
Answer

Answer
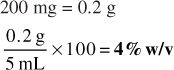
Dilutions
In dilution problems, one medication of a given concentration is being diluted with either an inert solvent or another medication solution that is not of interest in determining the final concentration of the original medication. So, for instance, dextrose 10% can be diluted with water or with saline where even though the saline contains sodium chloride, it does not contain dextrose so it is nothing more than a solute.
A complex dilution would occur if the 10% dextrose was to be diluted with 5% dextrose solution. In this instance both the beginning product and the diluent contain dextrose. Most dilutions are simple dilutions not complex.
Simple dilution uses the equation: ClVl = C2V2 or ClWl = C2W2, where C is concentration in percentage, ratio strength, or any other form of expressing amount per unit volume; V is volume and W is weight. ClVl or C1W1 is the starting concentration and volume or weight of the original medication being diluted. C2V2 or C2W2 is the final concentration and volume or weight. Key to remember is that V2 and W2 are made up of V1 or W1 plus the amount of diluent.
When solving this equation there are just a few things to remember: the units of the concentration terms must be the same as should be the units of the volume or weight of the medication in question. Problems cannot be solved if mixed units are used in this equation. Also, the concentration terms do not have to be in the form of percentages (even though that is the most typical means of expressing concentration). Concentration can be expressed as any unit of weight per unit of volume.
< div class='tao-gold-member'>



