Composite Lymphoma
Definition
The meaning and use of the term composite lymphoma has been used inconsistently over the years. The term was coined by Custer in 1954 for a patient with two or more types of lymphoma, as defined histologically, involving the same or different organs (1). In 1977, Kim and colleagues (2) suggested restricting the definition of composite lymphoma to two or more histologic types of lymphoma involving the same anatomic site simultaneously, and this was the definition used in the Working Formulation (3) published in 1982. With the advent of molecular analysis, our understanding of some of these cases has greatly increased. Despite the apparent distinctive histologic appearance of the multiple components in composite lymphoma, in some cases these components are clonally related whereas in others they are clonally unrelated. Some pathologists have tended to restrict the term composite lymphoma to only those cases in which the components are thought to be clonally unrelated (e.g., B-cell and T-cell non-Hodgkin lymphoma in the same lymph node) (4,5). Others have suggested that the term be used in a purely descriptive manner for neoplasms that have multiple distinctive histologic components irrespective of a clonal relationship (4).
Composite lymphoma must be distinguished from discordant histology, at least from a conceptual point of view. A patient with discordant histology has two or more histologic types of lymphoma occurring at separate anatomic sites, either simultaneously or sequentially (6,7,8). Jaffe and colleagues (8) have referred to these patients as having simultaneous or sequential lymphomas, respectively. However, these definitions are not universally accepted, as the term composite lymphoma is used to include synchronous (simultaneous) and metachronous lymphomas in a given patient by some investigators (9).
For the remainder of this chapter, we will use the definition of composite lymphoma as stated in the Working Formulation (3): two or more histologically distinct types of lymphoma involving the same anatomic site. We further subdivide these cases at the pathogenetic level into clonally related and unrelated groups.
Pathogenesis
In daily clinical practice, it is unusual for the individual components of a case of composite lymphoma to be analyzed to determine their clonal relationship. For this reason, we agree with the suggestion of Mueller-Hermelink and colleagues (4) that the term composite lymphoma should be used descriptively, to designate a single anatomic site involved by two or more histologic types of lymphoma. Virtually any histologic type of lymphoma can be in combination with another type. For the purpose of this discussion, we have subdivided these combinations, somewhat arbitrarily, into four groups: (1) two types of non-Hodgkin lymphoma of the same lineage; (2) B-cell non-Hodgkin lymphoma combined with Hodgkin lymphoma; (3) two types of non-Hodgkin lymphoma of different lineages; in other words, B-cell lymphoma combined with T-cell lymphoma; and 4) T-cell non-Hodgkin lymphoma combined with Hodgkin lymphoma.
Based on earlier studies of composite lymphomas, as well as patients with simultaneous and sequential lymphomas, many of the combinations of non-Hodgkin lymphoma in group 1, two non-Hodgkin lymphomas of the same lineage have a high likelihood of being clonally related. For example, in a composite lymphoma with two components, low-grade follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL), the latter usually represents histologic transformation (or progression) with both components carrying the same clone (10,11). This occurrence is in keeping with the natural history of low-grade FL to acquire large cells, usually associated with a diffuse pattern, over time. Similarly, in patients who have both typical and blastoid variant mantle cell lymphoma, either as composite or sequential lymphoma, both components are often clonally related (12,13). In patients with B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) who develop DLBCL, so-called Richter syndrome, a clonal relationship has been shown relatively less frequently, in approximately 50% to 60% of patients (14). Most often, clonality studies have been done in patients with sequential CLL/SLL and DLCBL, with relatively few molecular studies of composite CLL/SLL and DLBCL. Patients with lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia (LPL/WM) can develop DLBCL, usually sequentially, or rarely as a part of composite lymphoma (15). This is also true for patients with extranodal, nodal and splenic marginal zone B-cell lymphomas (16,17). Relative few cases of LPL/WM or the various types of marginal zone B-cell lymphoma have been assessed at the molecular level, with a subset of studies proving a clonal relationship between the indolent lymphoma and DLBCL (18,19,20,21). Patients with clinically indolent T-cell lymphomas, such as mycosis fungoides, can develop large T-cell lymphomas that may be CD30+ (22). These can be detected as composite lymphoma involving either skin or lymph node. A clonal relationship has been shown in a small number of these cases (23).
In group 2, B-cell non-Hodgkin lymphoma and Hodgkin lymphoma, the discordant histologic appearances of the components might lead one to presume a lack of a clonal relationship. However, during the past two decades it has become clear that the Reed-Sternberg and Hodgkin cells of Hodgkin lymphoma are almost always of B-cell lineage and that B-cell non-Hodgkin lymphoma and Hodgkin lymphoma are more closely related than was appreciated in the past. Patients with nodular lymphocyte-predominant Hodgkin lymphoma are known to have an increased risk of developing diffuse large B-cell lymphoma (24), and both the Hodgkin lymphoma and non-Hodgkin lymphoma are often clonally related. Patients with non-Hodgkin lymphoma have an increased incidence of developing classical Hodgkin lymphoma and vice versa, further suggesting a close relationship (25). In a subset of patients who had both non-Hodgkin lymphoma and classical Hodgkin lymphoma, either as composite lymphoma or sequentially, both neoplasms were shown to be derived from a common B-cell germinal center cell (26,27,28). Furthermore, the molecular data suggest that each component of these composite lymphomas arose
independently, and the non-Hodgkin lymphoma did not transform into classical Hodgkin lymphoma or vice versa (26,27). However, it must be emphasized that not all cases of composite non-Hodgkin lymphoma and Hodgkin lymphoma are clonally related.
independently, and the non-Hodgkin lymphoma did not transform into classical Hodgkin lymphoma or vice versa (26,27). However, it must be emphasized that not all cases of composite non-Hodgkin lymphoma and Hodgkin lymphoma are clonally related.
TABLE 70.1 Hypotheses to Explain the Pathogenesis of Clonally Unrelated Composite Lymphoma | |
|---|---|
|
In group 3 composite lymphoma, with T- and B-cell non-Hodgkin lymphoma components, the likelihood of a clonal relationship seems low. Group 4, T-cell non-Hodgkin lymphoma associated with Hodgkin lymphoma, is the least frequent group of composite lymphomas. T-cell non-Hodgkin lymphoma can be associated with either nodular lymphocyte-predominant Hodgkin lymphoma or classical Hodgkin lymphoma (5,29). The T-cell non-Hodgkin lymphoma components involved in these composite lymphomas have been classified as either peripheral T-cell lymphoma unspecified or mycosis fungoides. Molecular analysis of each component has shown no evidence of a clonal relationship in most of the few cases analyzed (5,29), and their pathogenesis may involve one or more of the listed hypotheses below. However, there appears to be a relationship between mycosis fungoides and classical Hodgkin lymphoma. At M. D. Anderson Cancer Center, patients with classical Hodgkin lymphoma have been shown to have a 290–fold increased risk of mycosis fungoides (30). Furthermore, one remarkable case has been reported of a patient with sequential mycosis fungoides, lymphomatoid papulosis, and classical Hodgkin lymphoma, all carrying the same T-cell clone (31).
A number of hypotheses have been suggested as possible explanations for composite lymphoma in which both components are clonally unrelated (including neoplasms in groups 1–4), as have been summarized by others (4,9,32). Currently, little experimental evidence supports any of the hypotheses listed in Table 70.1 and briefly summarized as follows:
Simple coincidence. This seems likely, at least for a subset of cases.
A common precursor cell that may give rise to both the B- and T-cell lymphoma components. To date, a common precursor cell of a composite B- and T-cell lymphoma has not been identified.
Patients may have a genetic predisposition to develop multiple lymphomas, thereby increasing the likelihood of developing two or more lymphomas involving one site.
Genetic instability might be involved. This may be acquired, and there is some evidence that MALT lymphomas exhibit genetic instability, possibly predisposing patients to a clonally related transformation or a second, clonally unrelated lymphoma. This genetic instability also could be inherited (related to the genetic-predisposition hypothesis).
Immunodeficiency, either inherited or acquired, may be involved (33). In patients with congenital immunodeficiency syndromes, both genetic predisposition and genetic instability also may be relevant. Patients with lymphoma also may have acquired immunodeficiency that predisposes them to second neoplasms, including another type of lymphoma
Immune dysregulation may be involved. Patients with autoimmune syndromes have an increased risk of non-Hodgkin lymphoma, Hodgkin lymphoma, and rarely both neoplasms (33,34). Methotrexate therapy for an autoimmune disorder also may predispose to a composite lymphoma (35). Patients with angioimmunoblastic T-cell lymphoma (AILT), a lymphoma known to be associated with immune system dysregulation, have an increased risk of DLBCL. The latter, which is often associated with Epstein-Barr virus (EBV) infection, can involve different sites or involve the same anatomic site as AILT, as a part of composite lymphoma (36,37).
Chemotherapy may be involved in the pathogenesis of composite lymphoma, with the second lymphoma in this case being sequential. Whether chemotherapy is genotoxic, directly involved in the genesis of a second lymphoma, or involved via therapy-associated immunosuppression (as in the immunodeficiency hypothesis) is unknown. Although therapy may be involved in the genesis of composite lymphoma in some patients, in many other patients who have never received therapy composite lymphomas also arise.
Exposure to a virus or other carcinogen could be involved in the development of two or more lymphoma types.
Factors secreted by one lymphoma type (for example, cytokines) may facilitate or stimulate development of a second lymphoma type.
Frequency And Clinical Findings
The lack of a consensus definition, the use of a number of classification systems over the years, and the infinite number of possible combinations of lymphoma types preclude making reliable statements regarding the epidemiologic attributes of patients with composite lymphoma. No true incidence data are available, but the relative frequency of composite lymphoma in various studies has ranged from 1% to 4.7%, depending on the classification system and method of detection (i.e., biopsy versus autopsy) (38). The age and the sex ratio of affected patients depends, in large part, on the epidemiologic findings associated with the various lymphoma types involved.
It seems reasonable to conclude that clonally related cases of composite lymphoma are more frequent than clonally unrelated cases, as the former occur more often than by chance alone. In the clonally related group, it also seems reasonable to expect that the incidence of composite lymphoma correlates with the overall frequency of the types of lymphoma involved, as well as with the frequency that these lymphomas undergo histological transformation. Within this framework, FL is a common type of lymphoma, and DLBCL in these patients is often clonally related. In two large studies of FL patients, the frequency of histologic transformation to DLBCL ranged from 11% to 28% (39,40). This frequency, of course, includes all patients who develop DLBCL, only a small subset of whom presumably had composite lymphoma with low-grade FL and DLBCL involving the same site.
From the clinical point of view, therapy is typically directed at the more aggressive component of a composite lymphoma. For example, when an indolent non-Hodgkin lymphoma is combined with either Hodgkin lymphoma or aggressive non-Hodgkin lymphoma, the therapy is directed to the Hodgkin
lymphoma or aggressive non-Hodgkin lymphoma, respectively.
lymphoma or aggressive non-Hodgkin lymphoma, respectively.
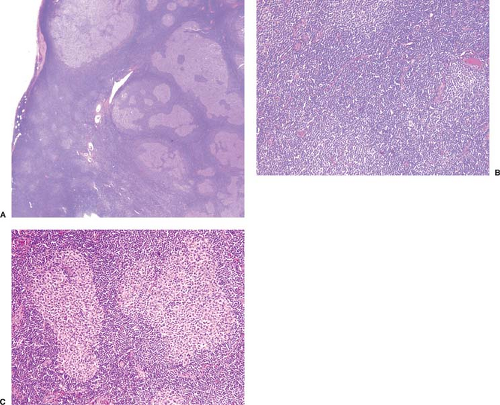 Figure 70.1. Composite lymphoma composed of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and follicular lymphoma. A: Low-power magnification showing that most of the lymph node is replaced by CLL/SLL with numerous pseudofollicles best seen in the lower left of the field. Neoplastic follicles are seen at the upper and right part of field. B: Higher-power magnification of CLL/SLL with three pseudofollicles in this field. C: Higher-power magnification of two neoplastic follicles surrounded by CLL/SLL. The clonal relationship of these two components was not assessed in this case. Hematoxylin-eosin stains.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|