Analytic methods to diagnose cobalamin deficiency fall into two categories: measures of cobalamin amount, such
as cobalamin and holotranscobalamin (holo-TC) II assays; and measures of functional metabolic status, such as the metabolite biomarkers, methylmalonic acid (MMA) and homocysteine, or complex cellular metabolic indicators such as the deoxyuridine suppression test. When clinical signs of deficiency are evident, one test usually suffices as confirmation (
15), but in research and epidemiologic surveys, the frequent absence of clinical identifiers usually requires the application of more than one diagnostic biomarker (
16). Unfortunately, no diagnostic gold standard exists.
Serum Cobalamin
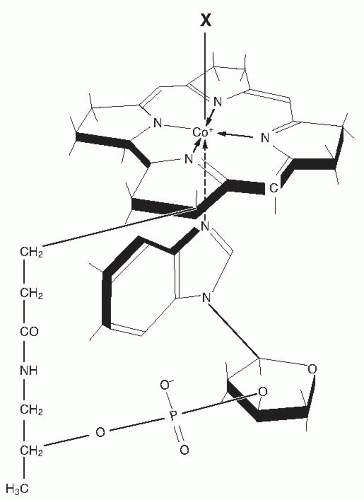
Cobalamin exists as methylcobalamin and other forms in serum (
17). Serum cobalamin is stable on long-term storage (although specific forms may be converted upon exposure to light) and can be assayed by various techniques. The earliest methods employed microorganisms, such as
Euglena gracilis and
Lactobacillus leichmannii, whose growth is proportional to the unknown sample’s cobalamin content (
17). The assays, now automated, are still considered the standard by some reference laboratories. Radioisotopic methods rely on competitive binding of the sample’s cobalamin by purified IF as the added cobalamin-binding protein; IF must not be contaminated with TC I, which also binds nonfunctional corrinoids and causes falsely high cobalamin results in samples with high levels of such corrinoids. Immunoenzymatic chemiluminescence techniques using anti-IF antibody to capture IF-complexed cobalamin now predominate in diagnostic use. These highly proprietary, automated assays have been less well defined and monitored than previous methods. They also appear susceptible to falsely normal results in some cobalamin-deficient sera (
18,
19), probably by failing to inactivate PA samples’ endogenous anti-IF antibody (
19). This selective error does not seem to affect normal sera, which complicates detection of the error; it may explain puzzling reports of normal cobalamin levels despite severe cobalamin deficiency (
16,
20).
The cutoff point between subnormal and normal serum cobalamin values varies from method to method and laboratory to laboratory (
16). Most laboratories use traditional decision limits (cut-points) of 200 to 250 ng/L (148 to 185 pmol/L) to define normality. The sensitivity of low cobalamin levels for cobalamin deficiency has been questioned but much depends on what kind of deficiency is considered (
15,
16). Sensitivity exceeded 95% in patients who had obvious clinical manifestations of deficiency, such as megaloblastic anemia or neurologic abnormalities (
16,
21,
22,
23). The lower the cobalamin level, the greater is the likelihood of clinically severe deficiency (
17,
24,
25), but exceptions exist (
26,
27). As with all biomarkers, diagnostic sensitivity decreases in subclinical conditions, and it is 38% to 39% in SCCD (
16). Low-normal cobalamin levels with associated metabolic abnormalities are not unusual in population surveys (
28,
29,
30), but metabolic abnormalities can be spurious sometimes (
16).
The cobalamin insensitivity in such surveys led some investigators to raise the cut-point for deficiency from the traditional 200 or 250 ng/L to 350 ng/L (258 pmol/L) or higher to ensure that no case of deficiency was
missed (
28). This change, adopted by many laboratories, instantly increased the frequency of diagnosed deficiency. The overdiagnosis it creates is substantial. For example, the 5.3% to 24.8% frequency of “abnormal” cobalamin values in four surveys became 40.5% to 71.7% in the unexceptional elderly populations (
15). More relevantly, reanalysis showed that only one third of the persons thus recategorized had MMA or homocysteine abnormalities, thus, making two thirds of the new diagnoses metabolically suspect; moreover, very few of the one third had clinical deficiency (
10,
16). Furthermore, the advantages of overdiagnosis remain unrealized because the health risks of SCCD and the benefits of intervention in such cases remain unproven.
As mentioned, low cobalamin levels’ specificity may be more limited than their sensitivity.
Table 27.1 lists the conditions associated with low serum cobalamin. The most notable causes of falsely low levels include pregnancy and folate deficiency (
17,
31), although true deficiency may accompany occasional cases. Substantive genetic influences on cobalamin levels are also becoming evident.
TCN1 mutations often cause TC I deficiency (
32,
33), and this deficiency may explain 15% of low cobalamin levels (
27) and thus may make it a more frequent cause of low serum cobalamin than PA.
Genetic influences can increase cobalamin levels, too, such as the unexplained moderately higher levels in homozygotes for a common polymorphism of the α-1,2 fucosyltransferase gene (
34,
35). Cobalamin levels are higher in Africans than in Asians or whites (
36), perhaps for genetic reasons. Spuriously, high cobalamin levels in medical settings most often result from renal failure or are idiopathic (
37). Autoantibodies to TCs can be therapy-induced (
38) or spontaneous (
39,
40), and they may explain 8% of elevated cobalamin levels (
41). Less frequent but often dramatic elevations accompany extremely high plasma TC I levels in chronic myelogenous leukemia and some cancers (
42).
Despite its limitations, cobalamin assay remains the first test of choice for now in patients suspected of cobalamin deficiency (
16,
43). Whatever the circumstances, including laboratory errors, incongruous laboratory results that conflict with a patient’s clinical picture must always be pursued further (
15). Additional tests of value when the diagnosis remains uncertain are discussed next. Cobalamin rise after therapy lacks specificity and, therefore, diagnostic value.
Methylmalonic Acid
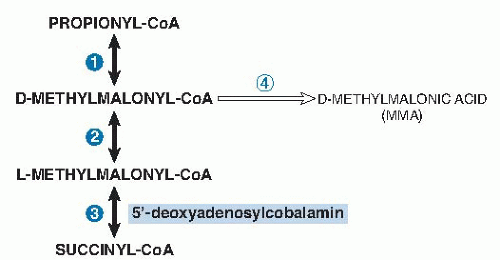
MMA accumulates in serum, and some MMA is excreted in the urine, when deoxyadenosylcobalamin-dependent methylmalonyl-CoA mutase activity is reduced (see
Fig. 27.3). MMA can be measured reliably by gas chromatography-mass spectrometry. Most laboratories define serum values higher than 280 nmol/L as abnormal, although cut-points have varied between 210 and 480 nmol/L (
16,
21,
44,
45). MMA is elevated in 98% of patients with PA who have clinically expressed cobalamin deficiency, often to levels exceeding 1000 nmol/L (
21,
22,
23,
46). MMA elevation reverses soon after cobalamin therapy (
22,
23).
MMA elevation is usually milder in SCCD because cobalamin stores are not severely depleted, and the sensitivity of high MMA, although not established because a gold standard comparator is lacking, is probably lower than in clinical deficiency (
16,
29,
30,
47). Specificity is clearly superior to that of homocysteine because folate status does not affect MMA (
22,
23,
46). However, major known influences on MMA include, in order of frequency, glomerular filtration (even a minimal decrease raises serum MMA), cobalamin status, age, and perhaps sex (
45,
48), and they explain only 16% of the MMA variation (
45). Asymptomatic babies can have moderate MMA elevation that remits spontaneously after the first year of life (
49). The cause is unknown, but the association with mild homocysteine and cobalamin changes (neither of which usually reach abnormality) and their improvement after cobalamin treatment raise the possibility of relative cobalamin deficiency (
50).
Many experts consider MMA the best metabolic test available to confirm cobalamin deficiency, and normal MMA values provide strong evidence against deficiency. However, MMA cannot be the diagnostic gold standard because its specificity is undefined (
16). It is particularly
unclear what mild MMA elevations without any other abnormalities mean (
16,
51,
52). The improvement of many isolated MMA elevations after cobalamin is given, which suggests that these elevations represented mild SCCD (
23,
30,
51). However, normal MMA levels often decline after cobalamin therapy, too—a finding suggesting regression to the mean or supersaturation of methylmalonyl-CoA mutase by the cobalamin as alternative explanations. A noteworthy longitudinal study of 432 patients left untreated over 1 to 3.9 years observed that 44% of isolated mild elevations of MMA improved spontaneously and only 16% progressed (
53). Antibiotics sometimes reduce cobalamin-unresponsive MMA elevation (
23,
54)—a finding suggesting that increased propionate metabolism by some intestinal bacteria may directly elevate MMA without cobalamin deficiency.
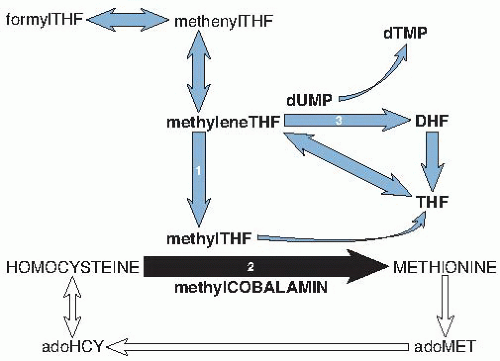
Homocysteine
Elevation of total homocysteine because of impaired methionine synthase activity is nearly as sensitive as MMA elevation for cobalamin deficiency. Sensitivity is 95% when cobalamin deficiency is clinically overt, and the homocysteine elevation is often striking (
23,
46). However, hyperhomocysteinemia has many causes (
55,
56), including preanalytic influences such as delayed blood sample processing or using serum instead of plasma, both of which promote artifactual release of red blood cell homocysteine. Renal status and folate status affect homocysteine more than does cobalamin status (
28,
55,
56), as observed best in countries that have not fortified the diet with folic acid (
48). The relative impact of cobalamin status on homocysteine rises in the aged, who have high rates of cobalamin deficiency. Other important influences on homocysteine include sex, genetic polymorphisms (especially methylene THF reductase), drugs, alcohol abuse, lifestyle factors, and disorders of homocysteine transsulfuration (
55,
56). Cut-points for homocysteine results have varied widely, and this affects case definition. Plasma levels lower than 10 µmol/L are considered optimal, but many laboratories use cut-points of 12 to 14 µmol/L in adult men and 10 to 12 µmol/L in premenopausal women.
Homocysteine is more reliable than cobalamin and is as reliable as MMA in monitoring therapeutic response in cobalamin deficiency; both homocysteine and MMA elevation respond to cobalamin but not to folic acid (
22,
23).
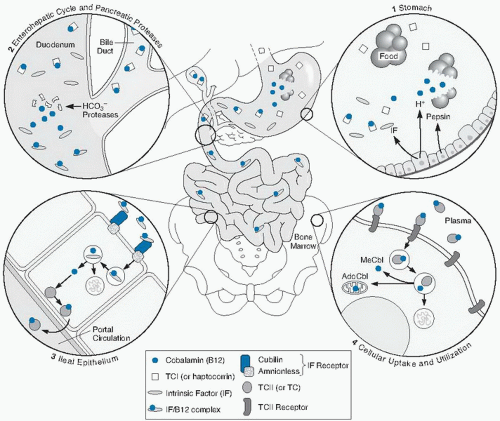
Holotranscobalamin II
Lindemans et al (
57) first suggested that measuring only the serum cobalamin attached to TC II, the carrier that promotes cellular uptake of cobalamin, may enhance diagnostic specificity and sensitivity of cobalamin testing. Holo-TC II, the TC II with attached cobalamin, originates in the ileal cell but may also have renal origins. Because holo-TC II is taken up by cells rapidly, less than 20% to 30% of plasma cobalamin is in holo-TC II at any moment; the remainder is carried by TC I, which does not promote specific cellular uptake (
42).
Holo-TC II assays are now accurate, and a fully automated method is commercially available (
58). As with other cobalamin-related biomarkers, holo-TC II cut-points vary widely between 19 and 50 pmol/L (
16). Direct comparisons show a minor advantage for holo-TC II over (total) cobalamin; areas under the curve by receiver operator curve analyses varied between 0.75 and 0.90 for holo-TC II versus between 0.72 and 0.85 for cobalamin (
16). The claim that holo-TC II does not decline spuriously like cobalamin in pregnancy and therefore permits better characterization of cobalamin status in pregnant women (
59) may be premature: an unnoticed holo-TC II rise actually followed delivery (prenatal levels were not determined)—a finding suggesting that holo-TC II had probably indeed declined during pregnancy (
16). Most holo-TC II studies have involved large amorphous groups defined almost solely by their MMA levels, whose specificity is itself uncertain. Clinical or absorptive status was rarely assessed, and disagreements between holo-TC II and cobalamin results were rarely explored (
16). One of the few clinical studies of patients with mild clinically expressed deficiency did not find holo-TC II to be superior to cobalamin in predicting response to therapy (
60).
Controversy persists about holo-TC II because very little is known about other influences on it. Renal failure can produce striking holo-TC II elevation (
37), but preliminary reports of many cobalamin-unrelated influences on holo-TC II such as alcohol abuse, folate deficiency, myelodysplasia, and Gaucher disease still await clarification (
16,
61). Moreover, holo-TC II levels lower than control values in cobalamin-repleted patients with PA (
62) suggest that cobalamin absorption affects holo-TC II independently of metabolic status. Such dual influences could introduce diagnostic nonspecificity; for example, it seems possible that even transient dietary change or malabsorption (e.g., drug induced) lasting only several days or weeks and unaccompanied by and unlikely to evolve into cobalamin deficiency might cause low holo-TC II levels. These and other influences may explain isolated holo-TC II elevations that have heretofore been attributed to an unusual sensitivity of holo-TC II for SCCD.