6 Clinical biochemistry and metabolism
Between 60 and 70% of all critical decisions taken in regard to patients in health-care systems in developed countries involve a laboratory service or result. This chapter describes disorders whose primary manifestation is in abnormalities of biochemistry laboratory results, or whose underlying pathophysiology involves disturbance in specific biochemical pathways. Discussion of diabetes mellitus and other endocrine disorders is to be found in Chapters 10 and 11.
WATER AND ELECTROLYTE DISTRIBUTION
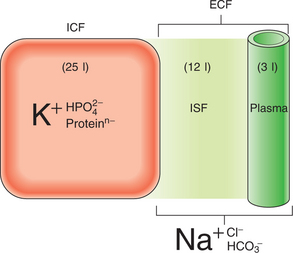
The dominant cation in the ICF is potassium, while in the ECF it is sodium (Fig. 6.1). Phosphates and negatively charged proteins constitute the major intracellular anions, while chloride and, to a lesser extent, bicarbonate dominate the ECF anions. An important difference between the plasma and interstitial ECF is that only plasma contains significant concentrations of protein.
The major force maintaining the difference in cation concentration between the ICF and ECF is the sodium–potassium pump (Na,K-activated ATPase) integral to all cell membranes. Maintenance of the cation gradients across cell membranes is essential for many cell processes, including the excitability of conducting tissues such as nerve and muscle. The difference in protein content between the plasma and the interstitial fluid compartment is maintained by the protein permeability barrier at the capillary wall. This protein concentration gradient contributes to the balance of forces across the capillary wall favouring fluid retention within the capillaries (the colloid osmotic, or oncotic, pressure of the plasma), thus maintaining an adequate circulating plasma volume.
DISORDERS OF SODIUM BALANCE
SODIUM DEPLETION (USUALLY ASSOCIATED WITH HYPOVOLAEMIA)
Aetiology includes the following factors:
Clinical features
Symptoms and signs of hypovolaemia are as follows:
Supportive biochemistry includes:
Management of sodium and water depletion
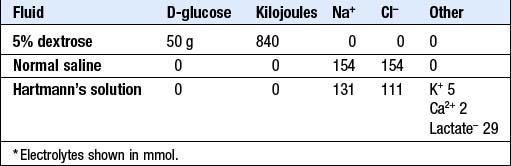
I.v. fluid therapy: Box 6.1 shows the daily maintenance requirements for water and electrolytes in a typical adult, and Box 6.2 summarises the composition of some widely available i.v. fluids. The choice of fluid and the rate of administration will depend on the clinical circumstances, as assessed at the bedside and from laboratory data.
6.1 BASIC DAILY WATER AND ELECTROLYTE REQUIREMENTS ![]()
| Requirement per kg | Typical 70 kg adult | |
|---|---|---|
| Water | 35–45 ml/kg | 2.5–3.0 l/day |
| Sodium | 1.5–2 mmol/kg | 100–140 mmol/day |
| Potassium | 1.0–1.5 mmol/kg | 70–100 mmol/day |
NB for Na and K, mmol = meq
SODIUM EXCESS (USUALLY ASSOCIATED WITH HYPERVOLAEMIA)
Causes of sodium and water excess in clinical practice include:
Peripheral oedema is the most common physical sign associated with these conditions (p. 174) although it is not usually a feature of Conn’s syndrome.
Management
The management of ECF volume overload involves:
DISORDERS OF WATER BALANCE
Disturbances in body water metabolism, in the absence of changes in sodium balance, manifest principally as abnormalities of plasma sodium concentration, and hence of plasma osmolality. The main consequence of changes in plasma osmolality, especially when rapid, is altered cerebral function. This is because, when extracellular osmolality changes abruptly, water flows rapidly across cell membranes with resultant cell swelling (during hypo-osmolality) or shrinkage (during hyperosmolality). Cerebral cell function is very sensitive to such volume changes, particularly during cell swelling where an increase in intracerebral pressure occurs due to the constraints posed by the bony skull, resulting in impaired cerebral perfusion.
HYPONATRAEMIA
Euvolaemic (water retention alone, i.e. ‘dilutional’): Primary polydipsia, SIADH (Box 6.3).
6.3 SYNDROME OF INAPPROPRIATE ANTIDIURETIC HORMONE SECRETION (SIADH): CAUSES AND DIAGNOSIS ![]()
Investigations
Plasma and urine electrolytes and osmolality (Box 6.4) are usually the only tests required to classify the hyponatraemia.
6.4 URINE Na AND OSMOLALITY IN THE DIFFERENTIAL DIAGNOSIS OF HYPONATRAEMIA* ![]()
| Urine Na (mmol/l) | Urine osmolality (mmol/kg) | Possible diagnoses |
|---|---|---|
| Low (<30) | Low (<100) | Primary polydipsia, malnutrition |
| Low | High (>150) | Salt depletion, hypovolaemia |
| High (>40) | Low | Diuretic action (acute phase) |
| High | High | SIADH |
* Note that intermediate urine results are of indeterminate significance and diagnosis depends on a comprehensive clinical assessment.
Management
Specific treatment should be directed at the underlying cause.
HYPERNATRAEMIA
Just as hyponatraemia represents a failure of the mechanisms for diluting the urine during free access to water, so hypernatraemia (plasma Na > 150 mmol/l) reflects an inadequacy of the kidney in concentrating the urine in the face of relatively restricted water intake. Similar to hyponatraemia, the causes of hypernatraemia may be classified based on the volume state of the patient.
Euvolaemic (water deficit alone): Diabetes insipidus (central or nephrogenic, p. 377).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree