
A. Endothelial Function and Regulation of Vascular Tone. Endothelial synthesis and release of vasoactive mediators are important elements in the regulation of vascular tone. Under physiologic conditions, local vascular pressure and flow are the primary stimuli for endothelial vasoactive substance release. Nitric oxide and prostacyclin are powerful vasodilators released by endothelial cells and both also inhibit platelet aggregation and thrombosis.
III. Components of the Systemic Circulation. The components of the systemic circulation are the arteries, arterioles, capillaries, venules, and veins.
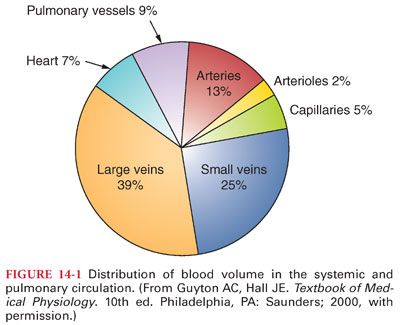
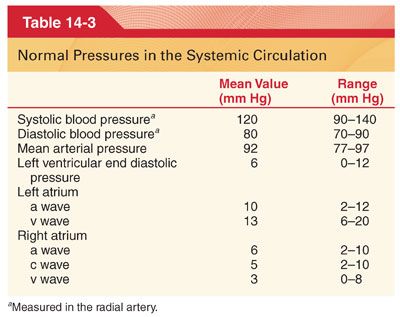
IV. Physical Characteristics of the Systemic Circulation. The systemic circulation contains about 80% of the blood volume, with the remainder present in the pulmonary circulation and heart (Fig. 14-1). The heart ejects blood intermittently into the aorta such that blood pressure in the aorta fluctuates between a systolic level of about 120 mm Hg and a diastolic level of about 80 mm Hg (Table 14-3) (Fig. 14-2). Standard physiologic monitors (heart rate, blood pressure, pulse oximetry, capnography) all serve as surrogate markers of organ perfusion and oxygenation.
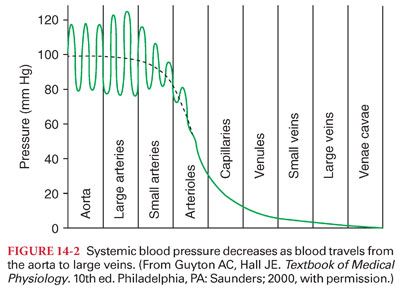
A. Progressive Declines in Systemic Blood Pressure. As blood flows through the systemic circulation, perfusion pressure decreases progressively to nearly 0 mm Hg by the time blood reaches the right atrium (see Fig. 14-2). The decrease in systemic blood pressure in each portion of the systemic circulation is directly proportional to the resistance to flow in the vessels.
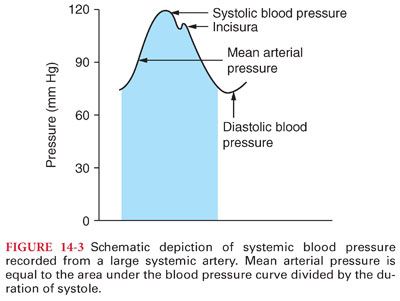
1. Pulse pressure in arteries reflects the intermittent ejection of blood into the aorta by the heart (see Table 14-3). The difference between systolic and diastolic blood pressure is the pulse pressure (Fig. 14-3).
2. Factors That Alter Pulse Pressure. The principal factors that alter pulse pressure in the arteries are the left ventricular stroke volume, velocity of blood flow, and compliance of the arterial tree.
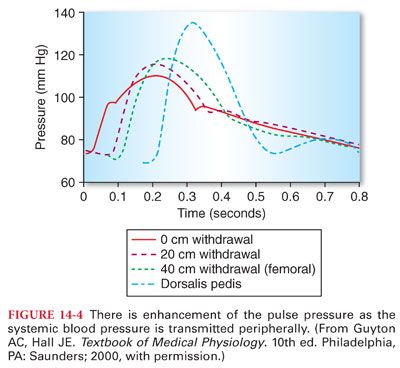
3. Transmission of the Pulse Pressure. There is often enhancement of the pulse pressure as the pressure wave is transmitted peripherally (Fig. 14-4). Augmentation of the peripheral pulse pressure must be identified whenever systemic blood pressure measurements are made in peripheral arteries (systolic pressure in the radial artery is sometimes as much as 20% to 30% higher than that pressure present in the central aorta, and diastolic pressure is often decreased as much as 10% to 15%). Mean arterial pressures are similar regardless of the site of blood pressure measurement in a peripheral artery.
4. Systemic Blood Pressure Measurement during and after Cardiopulmonary Bypass
a. Reversal of the usual relationship between aortic and radial artery blood pressures can occur during the late period of hypothermic cardiopulmonary bypass and in the early period after termination of cardiopulmonary bypass (Fig. 14-5).
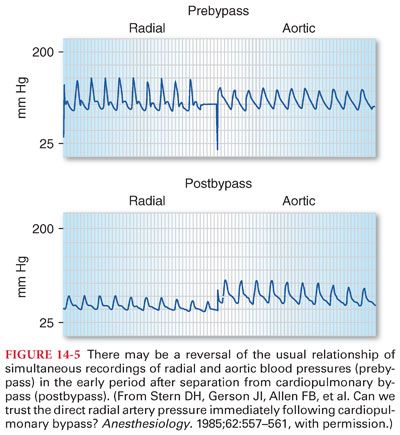
b. Failure to recognize this disparity may lead to an erroneous diagnosis and unnecessary treatment. Systemic blood pressure measured in the brachial artery is more accurate and reliable during the periods surrounding cardiopulmonary bypass.
5. Pulsus paradoxus is an exaggerated decrease in systolic blood pressure (>10 mm Hg) during inspiration in the presence of increased intrapericardial pressures (cardiac tamponade).
6. Pulsus alternans is alternating weak and strong cardiac contractions causing a similar alteration in the strength of the peripheral pulse (associated with digitalis toxicity, atrioventricular heart block, left ventricular dysfunction).
7. Electrical alternans is a phenomenon where the amplitude of the QRS complex changes between heart beats (cardiac tamponade, pericardial effusion).
8. Pulse Deficit. In the presence of atrial fibrillation or ectopic ventricular beats, two beats of the heart may occur so close together that the ventricle does not fill adequately and the second cardiac contraction ejects an insufficient volume of blood to create a peripheral pulse. In this circumstance, a second heart beat is audible with a stethoscope applied on the chest directly over the heart, but a corresponding pulsation in the radial artery cannot be palpated.
B. Measurement of blood pressure by auscultation uses the principle that blood flow in large arteries is laminar and not audible.
1. If blood flow is arrested by an inflated cuff and the pressure in the cuff is released slowly, audible tapping sounds (Korotkoff’s sounds) can be heard when the pressure of the cuff decreases just below systolic blood pressure and blood starts flowing in the brachial artery. Diastolic blood pressure correlates with the onset of muffled auscultatory sounds. The auscultatory method for determining systolic and diastolic blood pressure usually gives values within 10% of those determined by direct measurement from the arteries.
2. The width of the blood pressure cuff will affect measurements; ideally, the width of the blood pressure cuff should be 20% to 50% greater than the diameter of the patient’s extremity. If the cuff is too narrow, the blood pressure will be overestimated. If the cuff is too large, the blood pressure may be underestimated.
C. Right atrial pressure is regulated by a balance between venous return and the ability of the right ventricle to eject blood (normal right atrial pressure is about 5 mm Hg). Poor right ventricular contractility or any event that increases venous return (hypervolemia, venoconstriction) tends to increase right atrial pressure. Pressure in the right atrium is commonly designated the central venous pressure (CVP).
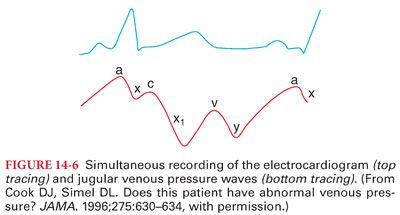
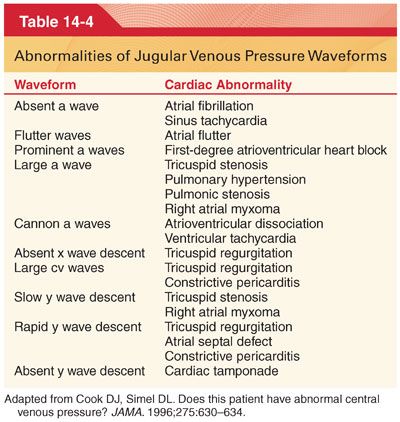
1. Jugular venous pressure mirrors the CVP. The normal jugular venous pressure reflects phasic changes in the right atrium and consists of three positive waves and three negative troughs (Fig. 14-6). Abnormalities of these venous waveforms may be useful in the diagnosis of various cardiac conditions (Table 14-4).
D. Effect of Hydrostatic Pressure
1. Pressure in veins below the heart is increased and that in veins above the heart is decreased by the effect of gravity (Fig. 14-7).
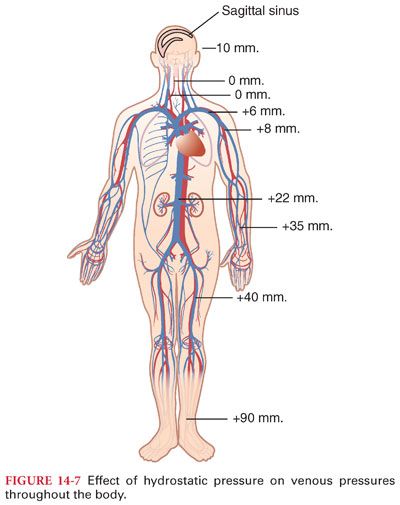
a. As a guideline, pressure changes 0.77 mm Hg for every centimeter the vessel is above or below the heart (pressure in the veins of the feet is 90 mm Hg).
b. Veins above the heart tend to collapse, with the exception being veins inside the skull, where they are held open by surrounding bone. As a result, negative pressure can exist in the dural sinuses and air can be entrained immediately if these sinuses are entered during surgery.
2. Hydrostatic pressure affects peripheral pressure in arteries and capillaries as well as veins (systemic blood pressure of 100 mm Hg at the level of the heart has a blood pressure of about 190 mm Hg in the feet).
E. Venous Valves and the Pump Mechanism. Valves in veins are arranged so that the direction of blood flow can be only toward the heart (in a standing human, the movement of the legs compresses skeletal muscles and veins so blood is directed toward the heart). If an individual stands immobile, the venous pump does not function and pressures in the veins and capillaries of the legs can increase resulting in leakage of fluid from the intravascular space (as much as 15% of the blood volume can be lost from the intravascular space in the first 15 minutes of quiet standing).
1. Varicose Veins
a. Valves of the venous system can be destroyed when the veins are chronically distended by increased venous pressure as occurs during pregnancy or in an individual who stands most of the day (result is varicose veins characterized by bulbous protrusions of the veins beneath the skin of the legs).
b. Venous and capillary pressures remain increased because of the incompetent venous pump, and this causes constant edema in the legs of these individuals. Edema interferes with diffusion of nutrients from the capillaries to tissues, so there is often skeletal muscle discomfort and the skin may ulcerate.
F. Reference Level for Measuring Venous Pressure
1. Hydrostatic pressure does not alter venous or arterial pressures that are measured at the level of the tricuspid valve (considered to be the level of the tricuspid valve). External reference points for the level of the tricuspid valve in a supine individual are about one-third the distance from the anterior chest and about one-fourth the distance above the lower end of the sternum.
2. For each centimeter below, the hydrostatic point adds 0.77 mm Hg to the measured pressure, whereas 0.77 mm Hg is subtracted for each centimeter above this point. The potential error introduced by measuring pressures above or below the tricuspid valve is greatest with venous pressures that are normally low.
3. The reason for lack of hydrostatic effects at the tricuspid valve is the ability of the right ventricle to act as a regulator of pressure at this site.
4. A venous pressure measurement in mm Hg can be converted to cm H2O by multiplying the pressure by 1.36, which adjusts for the density of mercury relative to water (10 mm Hg equals 13.6 cm H2O). Conversely, dividing the CVP measurement in cm H2O by 1.36 converts this value to an equivalent pressure in mm Hg.
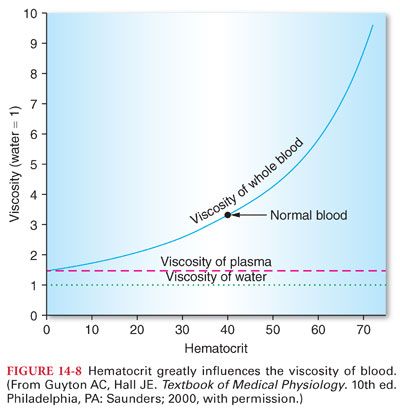
G. Blood Viscosity
1. The percentage of blood comprising erythrocytes is the hematocrit, which to a large extent determines the viscosity of blood (Fig. 14-8). When the hematocrit increases to 60% to 70%, viscosity of blood is increased about 10-fold compared with water, and flow through blood vessels is greatly decreased.
2. Plasma is considered extracellular fluid that is identical to interstitial fluid except for the greater concentrations of proteins (albumin, globulin, fibrinogen) in plasma. These greater concentrations reflect the inability of plasma proteins to pass easily through capillaries into the interstitial spaces. The presence of albumin creates colloid osmotic pressure, which prevents fluid from leaving the capillaries.
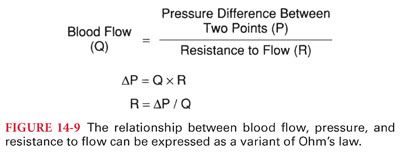
V. Determinants of Tissue Blood Flow. Tissue blood flow is directly proportional to the pressure difference between two points (not absolute pressure) and inversely proportional to resistance to flow through the vessel (Fig. 14-9). It is important to understand that resistance to blood flow cannot be measured but rather is a calculated value based on measurement of driving pressures and the cardiac output.
A. Vascular Distensibility
1. Blood vessels are distensible such that increases in systemic blood pressure cause the vascular diameter to increase, which in turn decreases resistance to blood flow. Conversely, decreases in intravascular pressure increase the resistance to blood flow.
2. Systemic blood pressure can eventually decrease to a level where intravascular pressure is no longer capable of keeping the vessel open (averages 20 mm Hg and is defined as the critical closing pressure). When the heart is abruptly stopped, the pressure in the entire circulatory system (mean circulatory pressure) equilibrates at about 7 mm Hg.
B. Vascular compliance is defined as the increase in volume (capacitance) of a vessel produced by an increase in intravascular pressure.
1. The compliance of veins is much greater than that of arteries. Enhancement of sympathetic nervous outflow to the blood vessels, especially the veins, decreases the dimensions to the circulatory system, and the circulation continues to function almost normally even when as much as 25% of the total blood volume has been lost.
2. Vasoconstriction or vasodilation refer to resistance changes in arterioles, whereas changes in the caliber of veins are described as venoconstriction or venodilation.
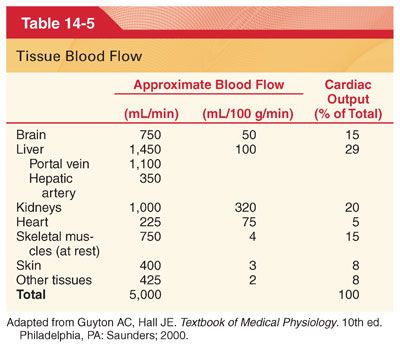
VI. Control of Tissue Blood Flow. Control of blood flow to different tissues includes local mechanisms, autonomic nervous system responses, and release of hormones (Table 14-5).
A. Local control of blood flow is most often based on the need for delivery of oxygen or other nutrients such as glucose or fatty acids to the tissues.
B. Autoregulation of blood flow is a local mechanism that controls blood flow in which a specific tissue is able to maintain a relatively constant blood flow over a wide range of mean arterial pressures. Autoregulatory responses to sudden changes in mean arterial pressure occur within 60 to 120 seconds.
C. Long-Term Control of Blood Flow
1. Long-term regulatory mechanisms that return local tissue blood flow to normal involve a change in vascularity of tissues.
2. Inadequate delivery of oxygen to a tissue is the stimulus for the development of collateral vessels. Neonates exposed to increased concentrations of oxygen can manifest cessation of new vascular growth in the retina. Subsequent removal of the neonate from a high-oxygen environment causes an overgrowth of new vessels to offset the abrupt decrease in availability of oxygen (retrolental fibroplasia).
D. Autonomic nervous system control of blood flow is characterized by a rapid response time (within 1 second) and an ability to regulate blood flow to certain tissues at the expense of other tissues.
1. Mass reflex is characterized by stimulation of all portions of the vasomotor center, resulting in generalized vasoconstriction and an increase in cardiac output in an attempt to maintain tissue blood flow.
2. Syncope. Emotional fainting (vasovagal syncope) may reflect profound bradycardia and skeletal muscle vasodilation such that systemic blood pressure decreases abruptly and syncope occurs. This phenomenon may occur in patients who have an intense fear of needles, resulting in syncope during placement of an intravenous catheter.
E. Hormone Control of Blood Flow. Vasoconstrictor hormones that may influence local tissue blood flow include epinephrine, norepinephrine, angiotensin, and arginine vasopressin (formerly known as antidiuretic hormone). Carbon dioxide also has an indirect vasoconstrictor effect because it stimulates the outflow of sympathetic nervous system impulses from the vasomotor center.
VII. Regulation of Systemic Blood Pressure. Systemic blood pressure is maintained over a narrow range by reciprocal changes in cardiac output and systemic vascular resistance. Because a greater portion of the cardiac cycle is nearer the diastolic blood pressure, it follows that mean arterial pressure is not the arithmetic average of the systolic and diastolic blood pressures. Mean arterial blood pressure is the most important determinant of tissue blood flow because it is the average, tending to drive blood through the systemic circulation.
A. Rapid-acting mechanisms for the regulation of systemic blood pressure involve nervous system responses as reflected by the baroreceptor reflexes, chemoreceptor reflexes, atrial reflexes, and central nervous system ischemic reflex. These reflex mechanisms respond almost immediately to changes in systemic blood pressure.
1. Baroreceptor Reflexes
a. Baroreceptors are nerve endings in the walls of large arteries in the neck and thorax, especially in the internal carotid arteries just above the carotid bifurcation and in the arch of the aorta.
b. These nerve endings respond rapidly to changes in systemic blood pressure and are crucial for maintaining normal blood pressure when an individual changes from the supine to standing position.
2. Chemoreceptor Reflexes
a. Chemoreceptors are cells that transduce chemical signals into nerve impulses. There are chemoreceptors located in the carotid bodies and aortic body. Each carotid or aortic body is supplied with an abundant blood flow through a nutrient artery so that the chemoreceptors are always exposed to oxygenated blood. When the systemic blood pressure, and thus the blood flow, decrease below a critical level, the chemoreceptors in the carotid body are stimulated by decreased availability of oxygen and also because of excess carbon dioxide and hydrogen ions that are not removed by the sluggish blood flow.
b. Chemoreceptors do not respond strongly until systemic blood pressure decreases below 80 mm Hg.
c. Chemoreceptors are more important in stimulating breathing when the Pao2 decreases below 60 mm Hg (ventilatory response to arterial hypoxemia). The ventilatory response to arterial hypoxemia is inhibited by subanesthetic concentrations of most of the volatile anesthetics (0.1 minimum alveolar concentration) as well as injected drugs such as barbiturates and opioids.
3. Central nervous system ischemic reflex occurs when blood flow to the medullary vasomotor center is decreased to the extent that ischemia of this vital center occurs. As a result of this ischemia, there is an intense outpouring of sympathetic nervous system activity, resulting in profound increases in systemic blood pressure. The central nervous system reflex response does not become highly active until mean arterial pressure decreases to less than 50 mm Hg and reaches its greatest degree of stimulation at systemic blood pressures of 15 to 20 mm Hg.
4. Cushing reflex is a central nervous system ischemic reflex response that results from increased intracranial pressure. When intracranial pressure increases to equal arterial pressure, the Cushing reflex acts to increase systemic blood pressure above intracranial pressure. Cushing’s triad is defined as having (a) hypertension, (b) bradycardia, and (c) irregular respirations (due to brainstem dysfunction). The latter is not often seen in this era, as most patients with severe intracranial hypertension are now mechanically ventilated.
5. Respiratory Variations in Systemic Blood Pressure
a. Systemic blood pressure normally varies by 4 to 6 mm Hg in a wavelike manner during quiet spontaneous breathing. This is due to increased venous return to the right heart during inspiration, which takes a few cardiac cycles to be transmitted to the left heart.
b. Positive pressure ventilation of the lungs produces a reversed sequence of blood pressure change because the initial positive airway pressure simultaneously pushes more blood toward the left ventricle.
c. Continuous or beat-to-beat monitoring of the changes in arterial blood pressure, pulse pressure, and stroke volume occurring during mechanical ventilation may provide an indication of the patient’s ability to respond to volume administration with an increase in cardiac output (fluid responsiveness). Respiratory variation in these parameters of more than 12% to 15% generally indicates fluid responsiveness.
6. Heart Rate Variability
a. Variations in heart rate occur during normal respiration, whereby inspiration increases heart rate and expiration decreases it. Analysis of heart rate variability provides information regarding the integrity of the autonomic nervous system.
b. Low heart rate variability can be a manifestation of disease (myocardial infarction, heart failure, neuropathy) and occurs universally following the denervation that occurs during cardiac transplantation.
B. Moderately Rapid-Acting Mechanisms for the Regulation of Systemic Blood Pressure. There are at least three hormonal mechanisms that provide either rapid or moderately rapid control of systemic blood pressure (catecholamine-induced vasoconstriction, renin-angiotensin–induced vasoconstriction, vasoconstriction induced by arginine vasopressin).
C. Long-term mechanisms for the regulation of systemic blood pressure, unlike the short-term regulatory mechanisms, have a delayed onset but do not adapt, providing a sustained regulatory effect on systemic blood pressure. The renal–body fluid system plays a predominant role in long-term control of systemic blood pressure because it controls both the cardiac output and systemic vascular resistance.
VIII. Regulation of Cardiac Output and Venous Return. Cardiac output is the amount of blood pumped by the left ventricle into the aorta each minute (product of stroke volume and heart rate), and venous return is the amount of blood flowing from the veins into the right atrium each minute (cardiac output must equal venous return). Cardiac output for the average person weighing 70 kg and with a body surface area of 1.7 m2 is about 5 L per minute. This value is about 10% less in women.
A. Determinants of Cardiac Output
1. Venous return is the main determinant of cardiac output. Any factor that interferes with venous return can lead to decreased cardiac output. Hemorrhage decreases blood volume such that venous return decreases and cardiac output decreases. Acute venodilation, such as that produced by spinal anesthesia and accompanying sympathetic nervous system blockade, can so increase the capacitance of peripheral vessels that venous return is reduced and cardiac output declines.
2. Factors that increase cardiac output are associated with decreases in systemic vascular resistance (anemia decreases the viscosity of blood, leading to a decrease in systemic vascular resistance and increase in venous return).
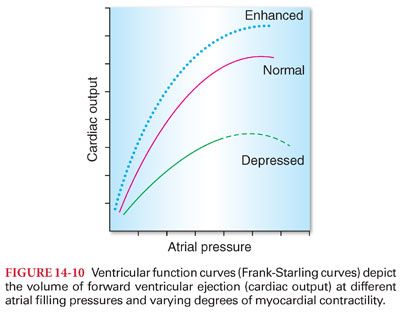
3. Ventricular function curves (Frank-Starling curves) depict the cardiac output at different atrial (ventricular end diastolic) filling pressures (Figs. 14-10 and 14-11). Clinically, ventricular function curves are used to estimate myocardial contractility.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


