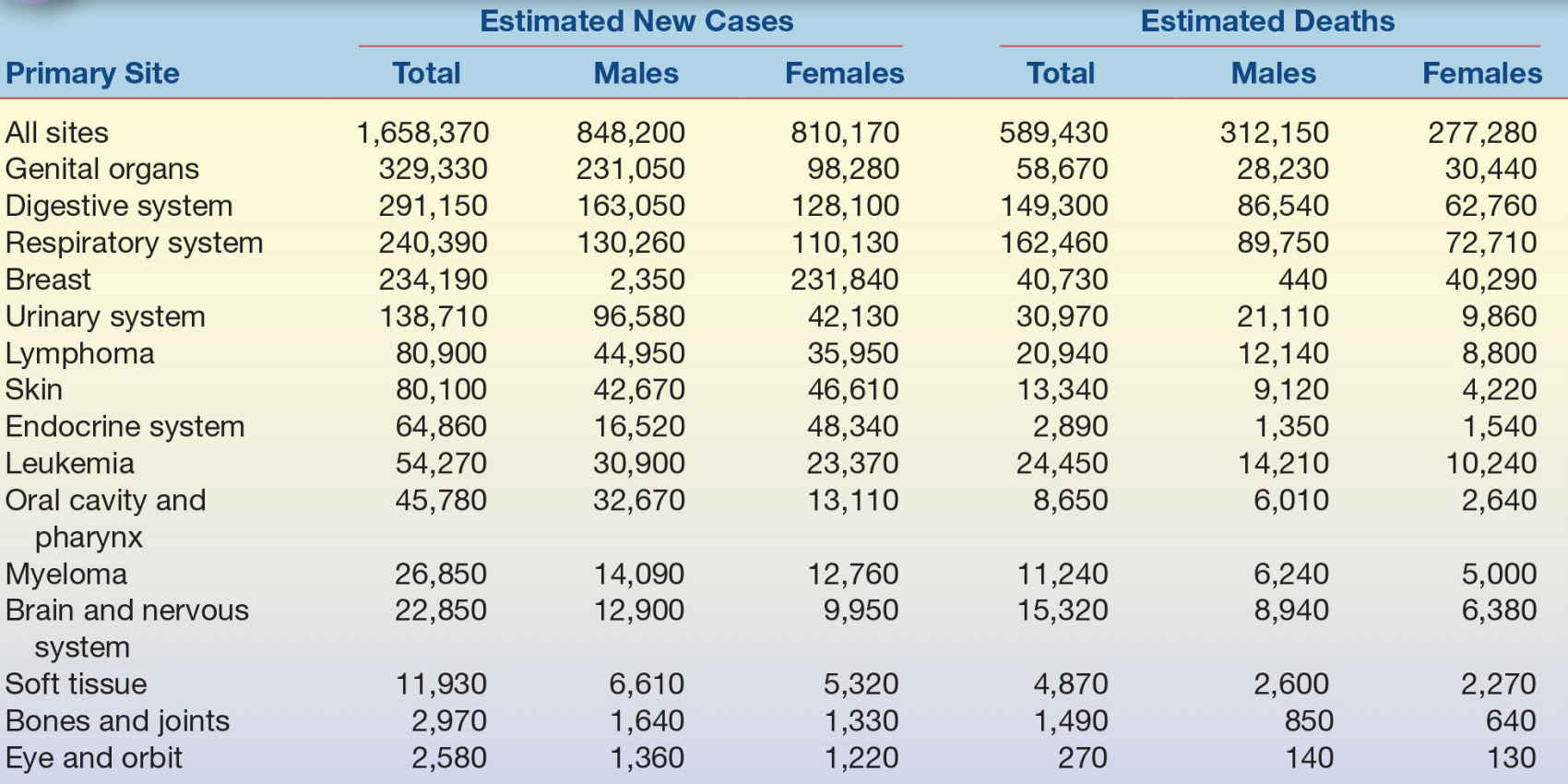
Cancer remains the second leading cause of mortality in developed countries, accounting for 23% of deaths in the United States and approximately 3 million deaths/year globally. Approximately 42% of males and 38% of females will develop invasive cancer in their lifetime; males have a lifetime risk of dying from cancer of 23%, whereas females have a 19% risk.1 Cancer is a broad term used to describe more than 200 different malignancies that affect more than 50 tissue types. Despite considerable efforts to reduce the incidence of malignancies, it is estimated that there were 1.6 million new cases of cancer in the United States in 2015 (Table 32.1; see additional current global cancer statistics at http://globocan.iarc.fr/factsheet.asp).
TABLE 32.1 Estimated New Cases of Cancer and Deaths From Cancer in the United States
Biologically, cancer refers to the uncontrolled growth of cells that often forms a solid mass or tumor (neoplasm) and spreads to other areas of the body. A complex combination of inherited and acquired genetic mutations lead to tumor formation (tumorigenesis) and spreading (metastasis) (for comprehensive reviews, see refs 2, 3). During tumorigenesis, mutations activate growth factors (e.g., epidermal growth factor) and oncogenes (e.g., K-ras), in combination with inhibition of apoptosis, tumor suppressor, and cell cycle regulation genes (e.g., BRCA1, p53, and cyclins). As cancer progresses toward metastasis, additional genetic changes are required, such as loss of cell adhesion proteins (e.g., β-catenin and E-cadherin) and activation of angiogenesis genes (e.g., vascular endothelial growth factor) (Fig. 32.1). An understanding of these genetic mechanisms is the basis for many current and future cancer treatments.
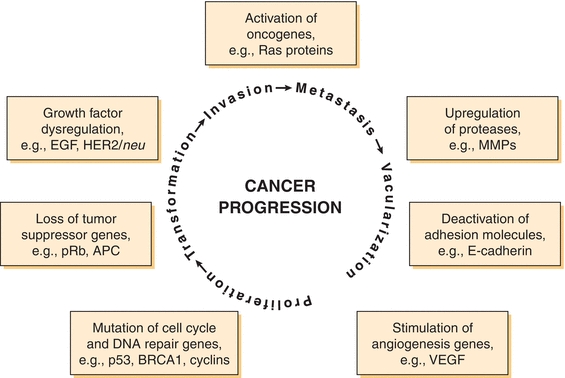
FIGURE 32.1 Genetic changes associated with cancer. A combination of acquired and/or hereditary defects causes tumor formation and metastasis. These processes begin with unregulated proliferation and transformation, followed by invasion and loss of cellular adhesion. A rich vascular supply of oxygen and nutrients is necessary to facilitate growth of a tumor larger than 100 to 200 μm. APC, familial adenomatous polyposis coli, mutated in colorectal cancers; BRCA1, breast cancer susceptibility gene; E-cadherin, adhesion molecule; EGF, epithelial growth factor; MMP, matrix metalloproteinase; p53, cell cycle regulator, mutated in 50% of cancers; pRB, retinoblastoma protein, mutated in many cancers; Ras, small G protein, mutated in many cancers; TIMP, tissue inhibitor of metalloproteinase; VEGF, vascular endothelial growth factor, drug target for inhibin of angiogenesis.
A combination of factors determines cancer severity and are used to classify its stage. Depending on the type of cancer, these factors include tumor size, histology, regional lymph node involvement, and presence of metastasis. For most solid tumors (e.g., breast, lung, and kidney), cancer is broadly classified (using roman numerals I to IV) into four stages (Fig. 32.2). Stages correlate with disease severity, where higher stages are indicative of larger tumors and/or significant spreading and severe systemic disease. With disease progression, both proliferation and metastasis occur at the expense of normal organ processes, which is usually the ultimate cause of cancer-associated morbidity and mortality.
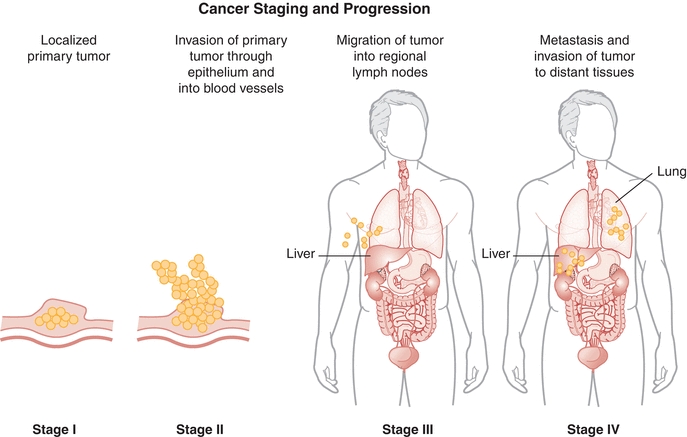
FIGURE 32.2 Generalized cancer staging and progression. Numerous factors are used in combination to define cancer stage; these include tumor size, extent of invasion, lymph node involvement, metastasis, and histologic assessments (basis for the TNM staging system). In this simplified diagram, stage is presented as a function of invasion and spreading regionally and to other tissues; the primary tumor is not shown.
TYPES OF TUMOR MARKERS
Cancer can be detected and monitored using biologically relevant tumor markers. Tumor markers are produced either directly by the tumor or as an effect of the tumor on healthy tissue (host). Tumor markers encompass an array of diverse molecules such as serum proteins, oncofetal antigens, hormones, metabolites, receptors, and enzymes.
A variety of enzymes are elevated nonspecifically in tumors (Table 32.2). These elevated enzymes are largely a result of the high metabolic demand of these proliferative cells. Accordingly, enzyme levels tend to correlate with tumor burden, making them clinically useful for monitoring the success of therapy. Serum proteins, such as β2-microglobulin and immunoglobulins, are also used to monitor cancer therapy (Table 32.3). β2-Microglobulin is found on the surface of all nucleated cells and can, therefore, be used as a nonspecific marker of the high cell turnover common in tumors. In hematologic malignancies such as multiple myeloma, immunoglobulins provide a relatively specific measure of plasma cell production of monoclonal proteins. In endocrine malignancies, hormones and hormone metabolites are widely used as specific markers of secreting tumors (Table 32.4). Hormones can be valuable in diagnosing neuroblastomas, as well as pituitary and adrenal adenomas. One of the first classes of tumor markers discovered was the oncofetal antigens. Oncofetal antigens such as carcinoembryonic antigen (CEA) and α-fetoprotein (AFP) are expressed transiently during normal development and are then turned on again in the formation of tumors (see Table 32.5 for use of oncofetal antigens). Other tumor markers include monoclonal defined antigens identified from human tumor extracts and cell lines. While these antibodies are directed toward specific carbohydrate or cancer antigens and are best used for monitoring treatment of tumors that secrete these epitopes (Table 32.6). Finally, receptors are used to classify tumors for therapy (Table 32.7). These “nonserologic” markers are outside the scope of the chapter, but they are an important example of the diversity of tumor markers. Prototypic examples of such a marker are estrogen and progesterone receptors and growth factors (HER-2), which are used to choose between endocrine and cytotoxic therapy; endocrine therapy, such as tamoxifen, typically is more effective in patient with ER- and PR-positive patients.
TABLE 32.2 Enzyme Tumor Markers
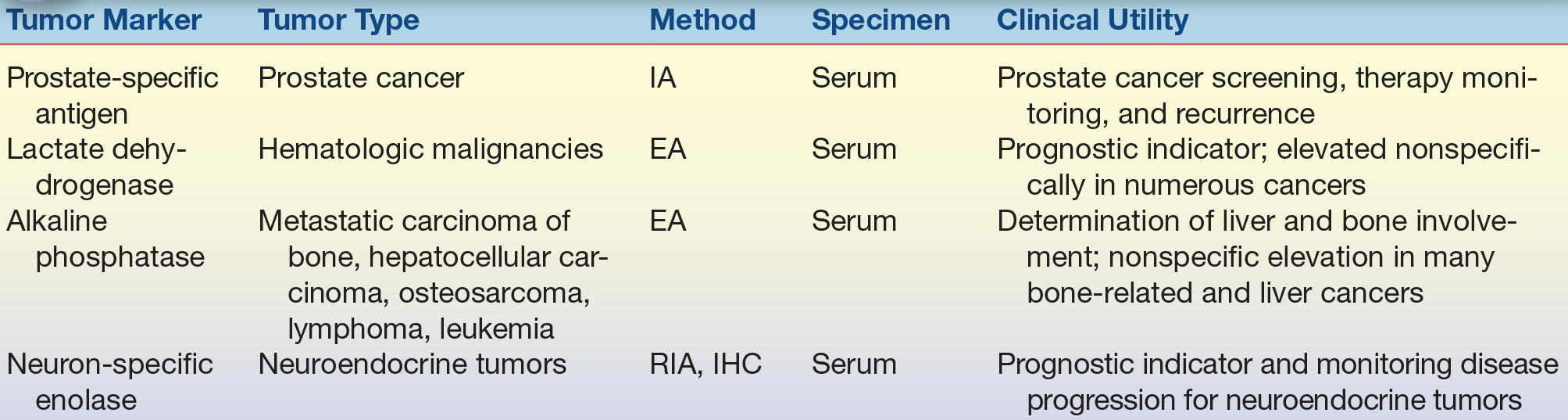
EA, enzyme assay; IA, immunoassay; IHC, immunohistochemistry; RIA, radioimmunoassay.
TABLE 32.3 Serum Protein Tumor Markers
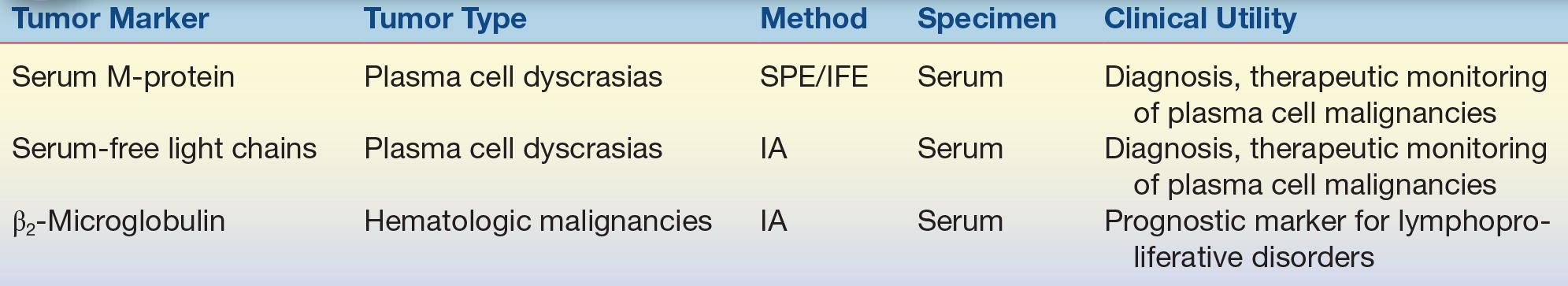
IA, immunoassay; IFE, immunofixation electrophoresis; SPE, serum protein electrophoresis.
TABLE 32.4 Endocrine Tumor Markers
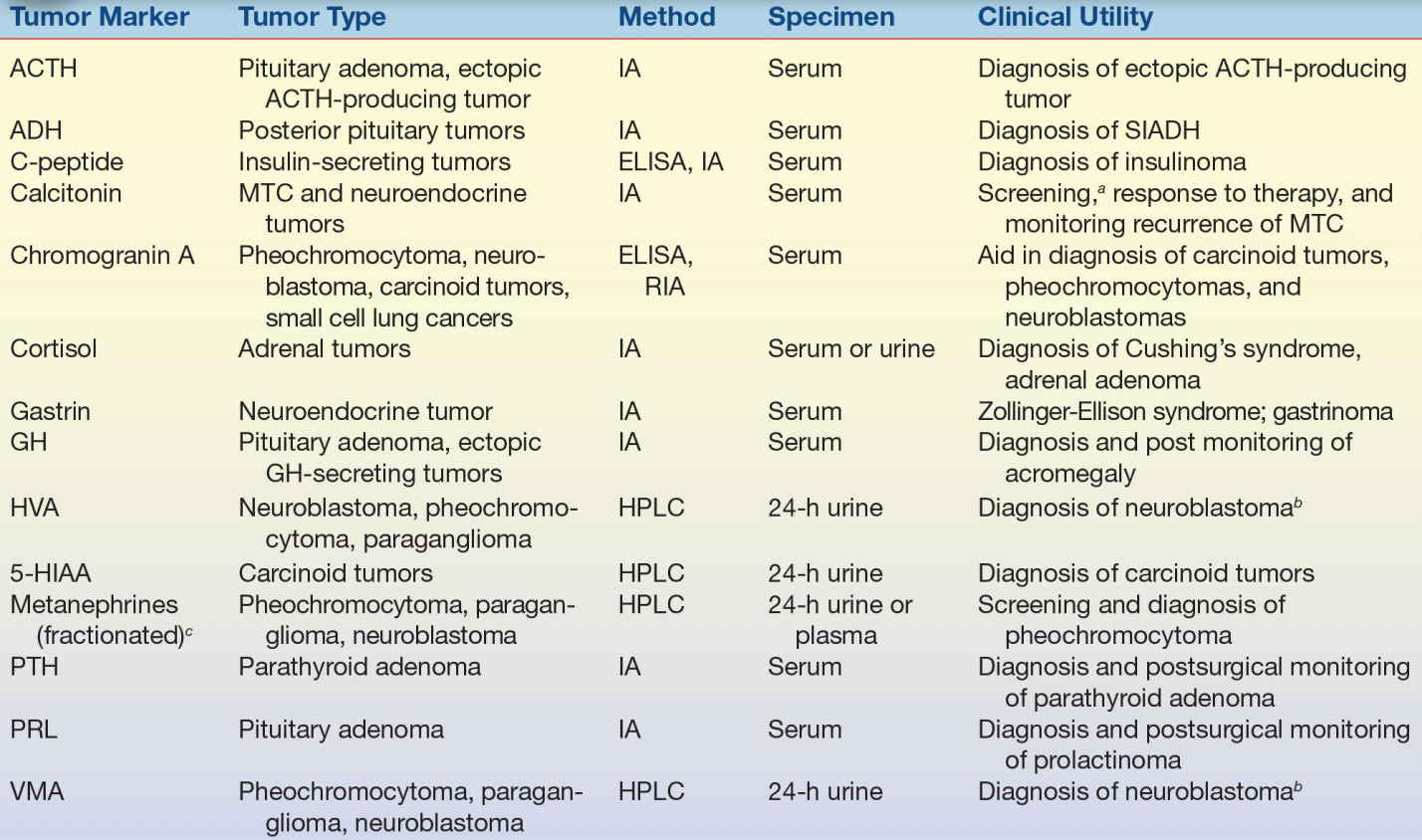
ACTH, adrenocorticotropic hormone; ADH, antidiuretic hormone; GH, growth hormone; HVA, homovanillic acid; 5-HIAA, hydroxyindoleacetic acid; PTH, parathyroid hormone; PRL, prolactin; VMA, vanillylmandelic acid; ELISA, enzyme-linked immunosorbent assay; HPLC, high-performance liquid chromatography; IA, immunoassay; LC-MS/MS, liquid chromatography–TANDEM mass spectrometry; MTC, medullary thyroid carcinoma; RIA, radioimmunoassay; SIADH, syndrome of inappropriate antidiuretic hormone secretion.
aScreening family members for MTC.
bHVA and VMA are used in combination for diagnosis of neuroblastomas.
cMetanephrine, normetanephrine.
TABLE 32.5 Use of Serum α-Fetoprotein and Human Chorionic Gonadotropin for Testicular Cancer Classification

AFP, α-fetoprotein; hCG, human chorionic gonadotropin.
TABLE 32.6 Carbohydrate and Cancer Antigen Tumor Markers
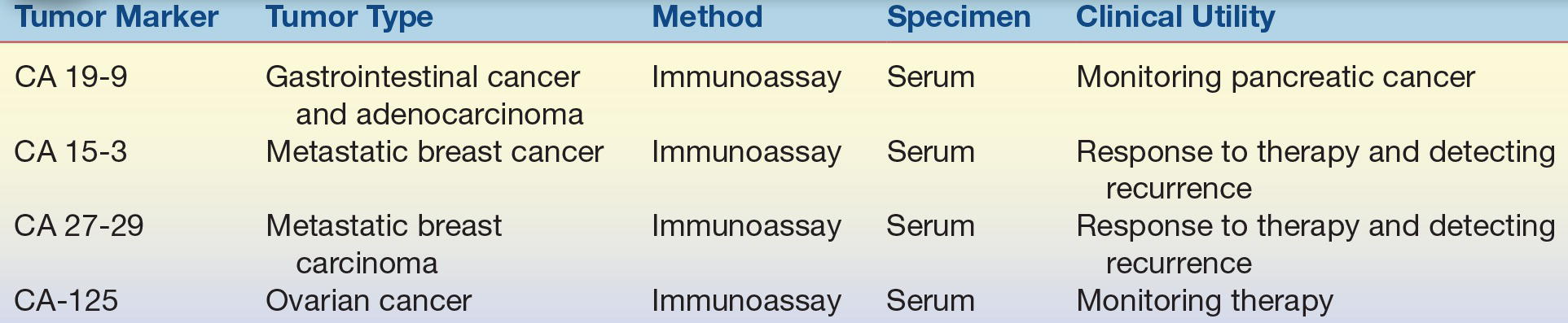
CA, cancer antigen.
TABLE 32.7 Receptor Tumor Markers
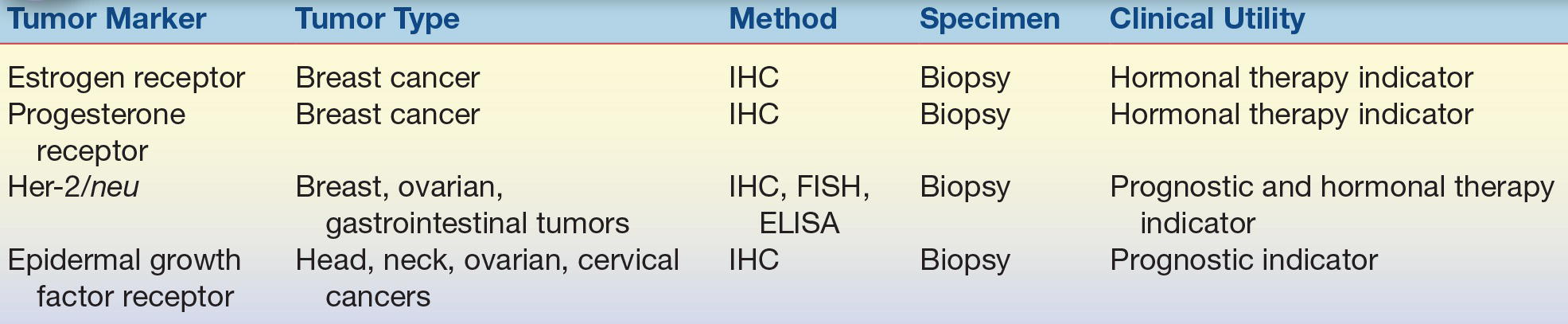
ELISA, enzyme-linked immunosorbent assay; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry.
Tumor markers are an invaluable set of tools that health care providers can use for a variety of clinical modalities. Depending on the marker and the type of malignancy, tumor markers for screening, diagnosis, prognosis, therapy monitoring, and detecting recurrence applications are in routine clinical use (Fig. 32.3).
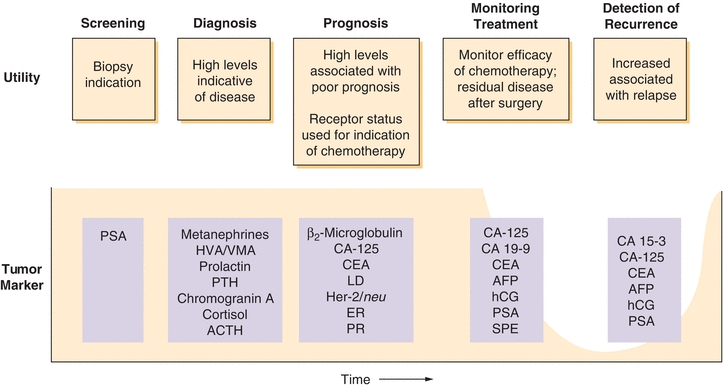
FIGURE 32.3 Tumor markers are used for screening, prognosis, treatment monitoring, and detecting recurrence of several types of cancer. Whereas few markers are used for screening, many are used to monitor therapy. Endocrine and hormone metabolite markers are often used to aid in diagnosis of secreting tumors. List is not comprehensive but provides examples of the most commonly used markers. Note: PSA screening remains controversial, see text.
APPLICATIONS OF TUMOR MARKER DETECTION
The ideal tumor marker would be tumor specific, absent in healthy individuals, and readily detectable in body fluids. Unfortunately, all of the presently available tumor markers do not fit this ideal model. However, numerous tumor markers have been identified that have a high enough specificity and sensitivity to be used on a targeted basis for aiding diagnosis, prognosis, detection of recurrence, and monitoring the response to treatment (Fig. 32.3). Clinically, tumor markers are used in combination with clinical signs, symptoms, and histology to facilitate clinical decision making.
Screening and Susceptibility Testing
With the possible exception of prostate-specific antigen (PSA),* no tumor marker identified to date can be used to effectively screen asymptomatic populations. This is because most of the clinically used tumor markers are found in normal cells and benign conditions in addition to cancer. Screening asymptomatic populations would therefore result in detection of false positives (patients without disease with detectable tumor marker), leading to undue alarm and risk to patients (e.g., unnecessary imaging, biopsy, and surgery). Presently, only a few tumor markers are used to screen populations with high incidence (targeted screening).
*Screening for prostate cancer remains controversial. Currently strategies focus on informed decision-making support to enable patients to weight the benefits of detecting disease early against the harms of overtreatment.
Susceptibility to cancer can be determined using molecular diagnostics in patients with breast, ovarian, or colon cancer by identifying germ-line mutations in patients with a family history of these diseases. Screening for susceptibility to breast and ovarian cancers is done by identifying germ-line BRCA1 and BRCA2 mutations. Similarly, familial colon cancers can be identified by the presence of the adenomatous polyposis coli (APC) gene. Since greater than 99% of people with familial APC develop colon cancer by the age of 40 years, prophylactic colectomy is routinely performed on APC+ patients. While gene testing can be done from blood samples, these are not really considered circulating tumor markers and therefore not discussed further.
Prognosis
Tumor marker concentration generally increases with tumor progression, reaching their highest levels when tumors metastasize. Therefore, serum tumor marker levels at diagnosis can reflect the aggressiveness of a tumor and help predict the outcome for patients. High concentrations of a serum tumor marker at diagnosis might indicate the presence of malignancy and possible metastasis, which is associated with a poorer prognosis. In other instances, the mere presence or absence of a particular marker may be valuable. Such is the case with some of the receptors used to base chemotherapeutic treatment in breast cancer as described above, where endocrine therapy is indicated only in the presence of given markers.
Monitoring Effectiveness of Therapy and Disease Recurrence
One of the most useful and common applications of tumor markers is monitoring therapy efficacy and detecting disease recurrence. After surgical resection, radiation, or drug therapy of cancer (chemotherapy), tumor markers are routinely followed serially. In patients with elevated tumor markers at diagnosis, effective therapy results in a dramatic decrease or disappearance of the tumor marker. If the initial treatment is effective, the appearance of circulating tumor markers can then be used as a highly sensitive marker of recurrence. Many tumor markers have a lead time of several months before disease would be detected by other modalities (e.g., imaging), allowing for earlier identification and treatment in cases of relapse.
LABORATORY CONSIDERATIONS FOR TUMOR MARKER MEASUREMENT
The unique characteristics and concentrations of tumor makers require special laboratory considerations. Two major considerations are the size and variability of the tumor marker concentration between different manufacturers due to the lack of harmonization and standardization. Lack of standardization makes comparison of serial patient results using different assays treacherous. There are multiple reasons why these assays are not comparable, including differences in antibody specificity, analyte heterogeneity, assay design, lack of standard reference material, calibration, kinetics, and variation in reference ranges. To most accurately monitor tumor marker concentrations in a patient, it is important to use the same methodology (or kit). It is also important to perform diligent quality control during lot changes. This includes a careful comparison of QC material and patient samples because detection of tumor markers can vary widely between reagent lots; this is particularly a concern where polyclonal antibodies are used as reagents (e.g., serum free light chains).
The other main consideration for tumor marker measurement is the wide range of concentrations encountered clinically. Tumor markers often vary in concentration by orders of magnitude, making accurate measurement challenging compared with routine chemistry analytes (e.g., concentration extremes for sodium are between 120 and 160 mmol/L, whereas human chorionic gonadotropin [hCG] may vary between 10 and 10,000,000 mIU/mL!). Handling these ranges requires careful attention to dilution protocols and the risk of antigen excess. These considerations are discussed in the context of specific methodology in the following sections.
Immunoassays
Immunoassays are the most commonly used method to measure tumor markers. There are many advantages to this method, such as the ability to automate testing and relative ease of use. Many tumor markers are amenable to automation and relatively rapid analysis using large immunoassay or integrated chemistry test platforms. However, there are some unique factors to be considered when using immunoassays to measure tumor markers including assay linearity, antigen excess (hook effect), and the potential for heterophile antibodies.
COMMON CANCER TERMS
Angiogenesis: Development of new blood vessels to supply oxygen and nutrients to cells
Apoptosis: Programmed cell death
Cell cycle: Phases of cell activity divided into G, S, and M (growth, DNA synthesis, and mitosis, respectively)
Neoplasm: Synonymous with “tumor,” it refers to uncontrolled tissue growth; it may be cancerous (malignant) or noncancerous (benign). Derived from Greek meaning “new formation.”
Oncogene: Encodes a protein that, when mutated, promotes uncontrolled cell growth
Tumor suppressor gene: Encodes a protein involved in protecting cells from unregulated growth
Linearity
The linear range is the span of analyte concentrations over which a linear relationship exists between the analyte and signal. Linearity is determined by analyzing (in replicates) specimens spanning the reportable range. Guidelines for this determination are outlined in the Clinical Laboratory Improvement Amendments (CLIA) guidelines for linearity.4 Samples exceeding the linear range, which is much more likely to occur in the detection of tumor markers, need to be systemically diluted to determine values within the reportable linear range. Dilutions must be done with careful consideration of the diluent and awareness of the risk of error if using manual calculations (it is common practice to have manual calculations reviewed by another individual). Excessively high tumor marker concentrations can result in falsely low measurements, a phenomenon known as antigen excess or hook effect.
Hook Effect
When analyte concentrations exceed the analytical range excessively, there is potential for antigen excess or hook effect. When very high antigen concentrations are present, capture and/or label antibodies can be saturated, resulting in a lack of “sandwich” formation and thus in a significant decrease in signal. The name hook effect refers to the shape of the concentration–signal curve when the reagents are saturated with excess antigen (Fig. 32.4). But the practical understanding of the hook effect is that it causes the actual tumor marker concentration to be grossly underestimated. If clinical suspicion is high for an elevated tumor marker, it can be identified by the laboratory with dilution and repeat testing. Samples displaying hook effect will yield higher (accurate) values on dilution (Fig. 32.4). This phenomenon typically only affects sandwich-type immunoassays.
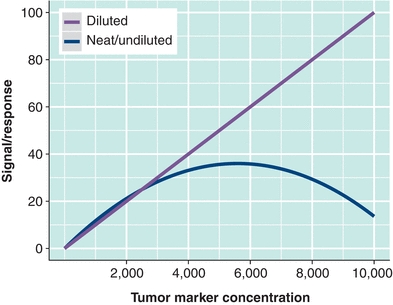
FIGURE 32.4 Hook effect (antigen excess) can occur with tumor markers because they may be found at very high concentrations. When reagents are depleted by excess antigen (the tumor marker), falsely low results may occur (represented by the “neat” curve). Dilution of samples can be used to detect and account for hook effect (represented by the “diluted” line).
Heterophile Antibodies
Significant interference can be seen in immunoassays if an individual has circulating antibodies against human or animal immunoglobulin reagents. A subset of heterophilic antibodies are human antianimal antibodies or human antimouse antibodies (HAMAs). HAMAs are most commonly encountered in patients who have been given mouse monoclonal antibodies for therapeutic reasons or who have been exposed to mice,5 but they may be idiopathic. In patients, these antibodies can cause false-positive or, less commonly, false-negative results by cross-linking the capture/label antibody (see Chapter 8). To confirm that heterophilic antibodies are present, samples may be diluted and the linearity of the dilutions is analyzed (similar to eliminating hook effect). Samples with heterophilic antibodies do not give linear results upon dilution. The presence of antianimal immunoglobulins can also be detected directly with commercial reagents. Nonimmune animal serum is often added to immunoassays to minimize the effects of heterophilic antibodies, and there are commercial blocking reagents that can be used to remove HAMAs. Many monoclonal therapeutic agents are now derived to include only fragments of an antibody so that patients do not develop heterophilic antibodies to the full antibody. In the laboratory, heterophile antibodies can be detected by investigating results that are inconsistent with history and clinical scenario.
Common Analytical Concerns Applied to Tumor Marker Immunoassays
Immunoassays for tumor markers can be affected by interference from icterus, lipemia, hemolysis, and antibody cross-reactivity in the same manner as other immunoassays. As with all automated tests, the potential for carryover with high levels of tumor marker analytes can also be a concern, leading to falsely elevated levels in patients if adequate washing steps are not included between patient samples.
High-Performance Liquid Chromatography
High-performance liquid chromatography (HPLC) is commonly used to detect small molecules, such as endocrine metabolites. With respect to tumor markers, HPLC is used to detect catecholamine metabolites in plasma and urine. Generally, there is an extraction process, by which the analytes of interest are separated from either plasma or urine. Extractions are applied to a column where they are separated by their physical characteristics (charge, size, and polarity). Catecholamines and catecholamine metabolites are used to help diagnose carcinoid tumors, pheochromocytoma, and neuroblastoma. Neuroblastoma is a common malignant tumor occurring in approximately 7.5% of children under 15 years of age. Neuroblastoma is diagnosed by the detection of high levels of plasma epinephrine, norepinephrine, and dopamine (catecholamines). Pheochromocytoma, a rare tumor associated with hypertension, is diagnosed by detecting elevated plasma metanephrines (along with urine vanillylmandelic acid and free catecholamines). Carcinoid tumors are serotonin-secreting tumors that arise from the small intestine, appendix, or rectum leading to a host of symptoms (carcinoid syndrome), including pronounced flushing, bronchial constriction, cardiac valve lesions, and diarrhea. The diagnosis of carcinoid tumors involves the detection of 5-hydroxyindoleacetic acid, which is a serotonin metabolite. In all of these cases, HPLC is used to detect the hormones and metabolites secreted by these tumors for diagnosis, therapeutic monitoring, and recurrence. HPLC is not subject to hook effect, lot-to-lot antibody variation, and heterophile antibodies but is more labor intensive and requires more experience and skill than automated immunoassays.
Immunohistochemistry and Immunofluorescence
While not found in circulation, it is important for laboratorians to be familiar with solid tissue tumor markers. These are identified in tissue sections typically from a fine-needle aspirate or biopsy samples. Specific antibodies (and the proper control antibodies) are incubated with tissue sections to detect the presence (or absence) of antigens using colorimetric or fluorescent secondary antibodies. In many ways, this is similar to detection by immunoassay, but the added value is the ability to determine whether the antigen in question is in a particular cell type (such as a tumor), in the specific subcellular location. A good example of the use of a tumor marker that is detected by immunohistochemistry is the identification of estrogen and progesterone receptors in breast cancer. When breast tumors are positive for estrogen and progesterone receptors at the cell surface, they tend to respond to hormonal therapy, while tumors lacking these receptors are treated with other chemotherapeutic modalities.6
Enzyme Assays
The detection of elevated circulating enzymes generally cannot be used to identify a specific tumor or site of tumor. One key exception to this is the PSA, which is in fact an enzyme; PSA is a serine protease of the kallikrein family, found in both diseased and benign prostate glands (it is also found in low concentrations in amniotic fluid, breast milk, and some other cancers). Before the widespread use of immunoassays and the discovery of oncofetal antigens, enzyme detection use was widespread. When cells die (autolysis/necrosis) or undergo changes in membrane permeability, enzymes are released from intracellular pools into circulation where they are readily detected. Examples of enzymes that have been used as tumor markers include alkaline phosphatase (bone, liver, leukemia, and sarcoma), lactate dehydrogenase (liver, lymphomas, leukemia, and others), and of course PSA (prostate). Enzyme activity assays (see also Chapter 13) are used to quantify all of these enzymes, with the exception of PSA, which is measured by immunoassay.
FREQUENTLY ORDERED TUMOR MARKERS
α-Fetoprotein
AFP is an abundant serum protein normally synthesized by the fetal liver that is reexpressed in certain types of tumors. This reexpression during malignancy classifies AFP as a carcinoembryonic protein. AFP is often elevated in patients with hepatocellular carcinoma (HCC) and germ cell tumors.
Regulation and Physiology
AFP is a 70-kD glycoprotein related to albumin that normally functions as a transport protein. Like albumin, it is involved in regulating fetal oncotic pressure. During development, AFP peaks at approximately one-tenth the concentration of albumin at 30 weeks of gestation. The upper normal limit for serum AFP is approximately 15 ng/mL (reference intervals are method dependent) in healthy adults. Infants initially have high serum AFP values that decline to adult levels at an age of 7 to 10 months.7
Clinical Application and Interpretation
AFP is used for the diagnosis, staging, prognosis, and treatment monitoring of HCC. Also known as hepatoma, HCC is a tumor that originates in the liver, often due to chronic diseases, such as hepatitis and cirrhosis. Patients with HCC frequently have elevated serum AFP. However, as with most tumor markers, AFP is not completely specific. For example, AFP can also be increased in benign conditions such as pregnancy and other nonmalignant liver disease, as well as other types of malignancies (e.g., testicular cancer—see below).
Although it is not widely used for screening in Europe and North America, AFP has been used to detect HCC in populations with high disease prevalence such as in China. When used for screening high-risk populations, AFP has a sensitivity ranging from 40% to 65% and specificity of 80% to 95% (at cutoffs ranging from 20 to 30 ng/mL).8 Very high levels of AFP (>500 ng/mL) in high-risk individuals are considered diagnostic of HCC.9 Several expert groups, including the National Comprehensive Cancer Network, National Academy of Clinical Biochemistry, and the British Society of Gastroenterology, now recommend that AFP be used in conjunction with ultrasound imaging every 6 months in patients at high risk for developing HCC. This includes patients with hepatitis B virus–induced and/or hepatitis C virus–induced liver cirrhosis.10
High levels of AFP in HCC are associated with poor prognosis and are exemplified in individuals who do not respond to therapy or have residual disease following surgery. Correspondingly, a decrease in circulating AFP levels after treatment is associated with prolonged survival rates. It is therefore recommended that serial measurements of AFP be used to monitor treatment and postsurgery in patients with HCC.
The other major use for AFP as a tumor marker is for classification and monitoring therapy for testicular cancer. Testicular cancer includes several subtypes broadly classified into seminomatous and nonseminomatous tumors. Seminomatous tumors form directly from malignant germ cells, whereas nonseminomatous tumors differentiate into embryonal carcinoma, teratoma, choriocarcinoma, and yolk sac tumors (endodermal sinus tumor).11 AFP is used in combination with β-human chorionic gonadotropin (β-hCG) to classify nonseminomatous tumors (Table 32.5). Serum AFP is also useful for tumor staging; AFP is increased in 10% to 20% of stage I tumors, 50% to 80% of stage II tumors, and 90% to 100% of stage III nonseminomatous testicular cancer. As with HCC, AFP can be used serially to monitor therapy efficacy and disease progression, where increases are indicative of relapse or resistance.
METHODOLOGY
AFP is measured using any of a variety of commercially available automated immunoassays. These are typically sandwich immunoassays relying on monoclonal or polyclonal antibodies directed toward different regions of AFP. Serial monitoring of AFP should be done using the same laboratory and assay method to ensure changes (or lack of change) are due to the tumor and not assay variation. As with other glycoproteins, AFP displays some heterogeneity where certain isoforms are preferentially produced by malignant cells; AFP isoforms differ in their glycosylation and sialyation. Antibodies against these isoforms produced by malignant cells may in the future be used to improve the specificity of AFP immunoassays.
Application and Pathophysiology
The primary applications of AFP as a tumor marker are for HCC and nonseminomatous testicular cancer. AFP is typically used as a marker to monitor therapy, detect residual tumor, or detect relapse; AFP is also used as part of maternal serum screening for neural tube defects and chromosomal abnormalities.
Cancer Antigen 125
Cancer antigen 125 (CA-125) was first defined by a murine monoclonal antibody raised against a serous ovarian carcinoma cell line.10 CA-125 may be useful for detecting ovarian tumors at an early stage and for monitoring treatments without surgical restaging.
Regulation and Physiology
CA-125 is expressed in the ovary, in other tissues of müllerian duct origin, and in human ovarian carcinoma cells. The CA-125 gene encodes a high molecular weight (200,000 to 1,000,000 kDa) mucin protein containing a putative transmembrane region and a tyrosine phosphorylation site.12 Although it is not usually found in serum, CA-125 may be elevated in patients with endometriosis, during the first trimester of pregnancy, or during menstruation.
Clinical Application and Interpretation
CA-125 is a serologic marker of ovarian cancer. Ovarian cancer accounts for approximately 3% of the newly diagnosed malignancies in women and is among the top five causes of cancer-related death (Table 32.1). Ovarian cancer includes a broad range of categories, including sex cord tumors, stromal tumors, germ cell tumors, and, most commonly, epithelial cell tumors. As with most other tumor markers, CA-125 should not be used to screen for ovarian cancer in asymptomatic individuals. However, CA-125 is elevated in a high percentage of ovarian tumors and is recommended as an annual test for women with a family or prior history of ovarian cancer. CA-125 levels also correlate with ovarian cancer stage. CA-125 is elevated in 50% of patients with stage I disease, 90% of patients with stage II, and more than 90% of patients with stage III or IV.
Other tumor markers for ovarian cancer are under development. Human epididymis protein 4 (HE4) is another Food and Drug Administration (FDA)–cleared test for ovarian cancer. HE4 offers improved specificity over CA-125, due to the presence of elevated CA-125 found in nonmalignant conditions, such as endometriosis. Tumor marker identification is still commonly published, with new markers on their way.
CASE STUDY 32.1
A 33-year-old man with a history of chronic liver disease presents with edema, abdominal pain, and recent weight loss. Laboratory examination reveals a low platelet count, hypoalbuminemia, and prolonged prothrombin time and partial thromboplastin time.
questions
1. Which tumor marker may aid in diagnosing this patient?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


