Circulating Cancer Cells
Myron R. Melamed
HISTORICAL OVERVIEW
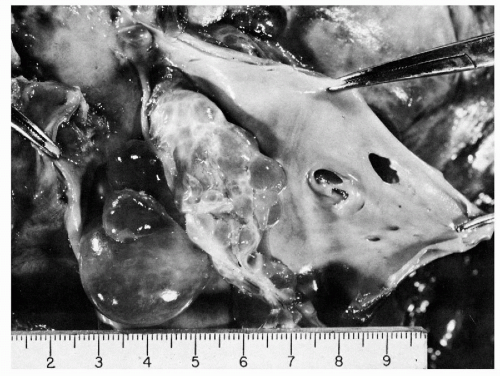
There is no doubt that cancer cells from almost any kind of malignant tumor can, and often do, enter into the blood stream and are, thereby, transported to distant organs where they may lodge and form metastases (Batson, 1940). Death from cancer in these patients is the result of metastatic dissemination. Visceral metastases were long known to be more common and patients’ survival shortened when venous invasion by malignant tumors was demonstrated (Brown and Warren, 1938; Sunderland, 1949; Grinnell, 1950; Collier et al, 1958; Friedell and Parsons, 1962). Goldmann (1906) reported grossly visible venous invasion by tumor in about 20% of 500 necropsies and microscopic invasion of veins in nearly 10% more (Fig. 43-1). Key references to the original 19th and early 20th century concepts of tumor cell embolization as a mechanism for dissemination of cancer may be found in historical reviews by Ewing (1940), Willis (1952), Coman (1953), West et al (1964), Griffiths and Salsbury (1963), and Watne et al (1966).
The number of cancer cells within the blood stream (excluding leukemias) is infinitesimal when compared with the number of normal blood cells. Thus, it is extraordinary to find cancer cells in routine blood smears, though such cases have been reported, as the so-called “carcinocythemia” (Finkel and Tishkoff, 1960; Goodall et al, 1961; Rappaport, 1966; Carey et al, 1976; Myerowitz et al, 1977; Dannaher et al, 1979; Ejheckam et al, 1979; Krause, 1979; Solanki et al, 1980; Gallivan and Lokich, 1984; Irie et al, 1985; Yam and Janckila, 1987; Lugassy et al, 1990; Maldonado et al, 1991; Sile et al, 1999; Rodriguez-Salas et al, 2000).
A systematic search for cancer cells in the circulating blood of cancer patients became a major research target in the 1950s and 1960s, as a possibly valuable predictor of disease progression and guide to therapy. The task of separating or concentrating these cells and giving them their proper identification proved to be a major technical problem, and interest in the research community waned over the next few years.
During the 1990s, technical advances in immunocy-to-chemistry and molecular biology reignited interest in the identification of circulating cancer cells in peripheral blood.
In this chapter, we summarize earlier efforts and report on the current status of these investigations.
In this chapter, we summarize earlier efforts and report on the current status of these investigations.
Older Studies
The earliest recorded description of free-floating cancer cells in a specimen of blood was by Ashworth in 1869. He reported finding pigmented tumor cells in direct smears of postmortem saphenous venous blood from a 38-year-old man who died with disseminated subcutaneous malignant tumors, presumably a malignant melanoma. Isolated observations of a similar nature were subsequently reported by Schleip (1906) and Ward (1913) in cases of gastric carcinoma, by Marcus (1917) in finger blood from a patient with lung cancer, by Loeper and Loeste in two patients with sarcoma (cited by Ewing, 1940), and by Quensel (1921) in the hearts’ blood from six of 50 cadavers with various malignant tumors.
In 1934, Pool and Dunlop carried out the first deliberate search for cancer cells in the blood from living patients. Their technique was crude: 5 ml of oxalated blood was hemolyzed with 15% acetic acid and the centrifuged sediment fixed with 10% formalin in alcohol, then embedded in paraffin and step-sectioned. They found “large spherical cells with hyperchromatic nuclei” in 17 of 40 cancer cases, but also in one patient with a benign gastric ulcer.
In 1954, Cole, Packard, and Southwick demonstrated cancer cells in the perfusate from mesenteric vessels of a resected segment of colon with carcinoma. In the following year, Fisher and Turnbull studied 25 consecutive patients with colorectal carcinoma undergoing surgical resection and found tumor cells in the blood and perfusate from mesenteric veins of 8 (32%). They suggested that tumor cells were dislodged by operative manipulation and Turnbull et al (1965) subsequently reported that ligation and division of the lymphovascular pedicle, before manipulation of the tumor, increased 5-year survival.
A study of major importance was the work of Engell in 1955, who reported in great detail on the incidence of cancer cells in regional venous and peripheral blood. He used 2 to 5 ml of heparinized blood in most cases, lysed the red cells with 1% saponin, and embedded the remaining cellular sediment in paraffin. In serial sections stained with hematoxylin and eosin, Engell found cancer cells in blood draining the tumor area of 63 of 107 colorectal carcinomas (59%), 6 of 8 cases of gastric carcinoma, 3 of 4 cases of lung cancer, and 3 of 6 cases of breast cancer. Peripheral blood from the cubital vein of 14 patients with advanced, inoperable carcinoma was examined, and cancer cells were found in 7, whereas in 70 patients with operable carcinoma, cancer cells were identified in only 10. Although cancer cells were more common in patients with more anaplastic tumors, there was no demonstrable relationship to the stage or to operative manipulation of the tumor. In a follow-up report 4 years later, Engell (1959) was unable to correlate the presence or absence of cancer cells in the peripheral blood with survival.
Engell’s work marked the beginning of a period of extraordinary interest in cancer cells in the blood. During little more than a decade after his second report, there were many other publications concerned with the technical problems of preparation and cytologic interpretation, as well as the actual results and their clinical correlation (Pruitt et al, 1958, 1963; Reiss, 1959). Because accuracy of the examination and interpretation of reported results depends on the quality of the preparation, a brief review of the preparatory methods used at that time is necessary.
Early Technical Methods
The great variety of techniques that had been proposed for processing peripheral blood specimens was probably a reflection of dissatisfaction with any one of them. Most widely used were the methods of Seal (1956, 1959, 1964), Roberts et al (1958), Long et al (1959), and Malmgren et al (1958). Their common purpose was the selection or concentration of any cancer cells that might be present in the blood. All of the methods involved removing the red blood cells and as many normal leukocytes as possible, while retaining cancer cell morphology and quantitative reproducibility.
Separation of the red cell mass was accomplished either by hemolysis, initially with water (Quensel, 1921), and later with acetic acid (Pool and Dunlop, 1934; Fisher and Fisher, 1959; Romsdahl et al, 1965), or by saponin (Engell, 1955; Nedelkoff et al, 1961; Ericksson, 1962). Enzymes, such as streptolysin-O, were used by Potter and Malmgren (1958). Sedimentation, usually with an accelerating factor such as fibrinogen (Buckley et al, 1950; Sandberg and Moore, 1957; Roberts et al, 1958), dextran (Alexander and Spriggs, 1960; deCarvalho, 1960; de Mello, 1963), phytohemagglutinin (Li and Osgood, 1949) or hemolymph heteroagglutinin (Watne et al, 1966), were also tried.
Separation of cell types may be facilitated by differential sedimentation or centrifugation after suspending the blood in a solution of albumen (Fawcett et al, 1950; McGrew, 1954; Roberts et al, 1958), gum acacia (Spear, 1948), or silicone of specific gravity adjusted between that of the heavier red blood cells (1.092-1.097) and the lighter cancer cells and lymphocytes (1.056-1.065) (Seal, 1959). Polyvinylpyrrolidone (PVP) and a detergent “wetting” agent were often added to prevent cell clumping and to keep platelets and cellular debris in suspension during centrifugation. Danielsson (1961) suggested using a spiral centrifuge for separation of the lighter cellular elements, but this offered little practical advantage for the relatively small volumes of blood usually examined. Polymorphonuclear leukocytes, which are heavier than lymphocytes and most cancer cells, were removed by differential centrifugation, or they were differentially lysed by streptolysin-O. None of these methods worked perfectly.
A technique for removing phagocytic leukocytes was introduced by Kuper et al (1961), who incubated the heparinized blood with carbonyl iron of approximately 3 μm particle size, and then removed the iron-containing cells by stirring the solution with a magnet. Buckman et al (1984) later introduced immuno-rosetting to select breast cancer cells. These methods were revisited in recent years (see below).
After processing the blood by any one, or several, of these methods, the final cell suspension was transferred to membrane filters (Seal, 1956) or centrifuged onto glass slides (Ericksson, 1962), or simply smeared on glass slides and stained. Membrane filters were more convenient and more popular and had the advantage of possibly better quantitation (Fig. 43-3). Much better cell detail was obtained by any of these cytologic methods than by stepsections of the cell sediment after paraffin embedding. The choice of stain was a matter of personal preference. Hematoxylin and eosin or the Papanicolaou stain was commonly used with all types of preparations; Wright-Giemsa or other hematologic stains, and the metachromatic fluorochrome acridine orange (de Mello, 1963) were generally restricted to smears on glass slides.
Best cell morphology was obtained by Roberts et al (1958,
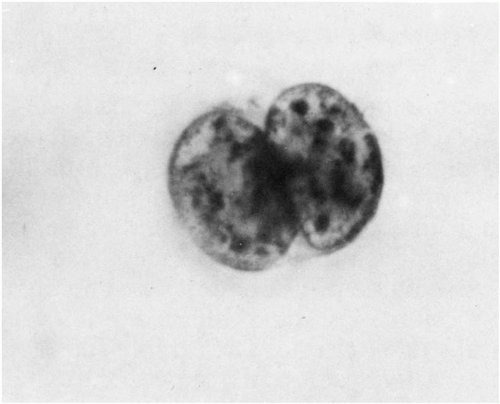
1961) using fibrinogen sedimentation to remove red blood cells, then concentrating any cancer cells in the supernatant plasma by centrifuging over an albumen solution of specific gravity 1.065. Most cancer cells collect and can be aspirated from the plasma-albumen interface, washed, streaked directly on glass slides, and stained with the Papanicolaou method (Fig. 43-2).
1961) using fibrinogen sedimentation to remove red blood cells, then concentrating any cancer cells in the supernatant plasma by centrifuging over an albumen solution of specific gravity 1.065. Most cancer cells collect and can be aspirated from the plasma-albumen interface, washed, streaked directly on glass slides, and stained with the Papanicolaou method (Fig. 43-2).
Direct separation of cells by size alone was undertaken using either a 10-μm pore membrane filter (West et al, 1964) or a 4.5- to 5-μm pore perforated plastic membrane sieve (Seal, 1964). The latter method gave technically good preparations, in addition to its great simplicity. A sample of anticoagulated blood was simply poured through the perforated plastic membrane, which retained the larger cells, including cancer cells. The membrane itself remained clear and transparent during staining and could be mounted on a glass slide.
Initial Observations
Recognition of Malignant Versus Benign Cells in Blood
Because of methods used to process the blood, cellular morphology was often suboptimal and poorly preserved cancer cells were difficult or impossible to distinguish from large immature hematopoietic cells. In some impeccable preparations, conventional criteria for recognition of epithelial cancer cells did apply, but this was only rarely the case. Consequently, the major source of discrepancies in reported results was due to differences in interpretation and classification of certain cells that are uncommonly found in ordinary blood smears (Ederer et al, 1965). Criteria for the differential diagnosis of cells likely to be confused with cancer cells in blood, have been described in detail by Sandberg et al (1959), Alexander and Spriggs (1960), McGrew and associates (1960, 1962, 1965), Scheinin and Koivuniemi (1962), Griffiths (1965), and the Circulating Cancer Cell Cooperative (Nadel and Goldblatt, 1965).
Megakaryocytes, endothelial cells, and immature hematopoietic cells that are rare in peripheral blood smears from individuals without hematologic disorders, are found with surprising regularity in the concentrates prepared for cancer cell studies.
Megakaryocytes
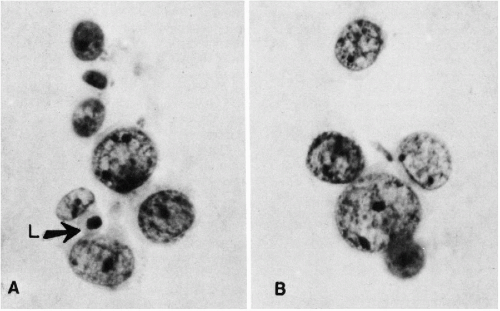
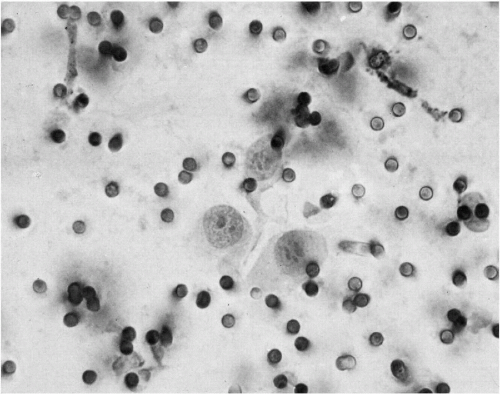
There are major differences in the morphology of megakaryocytes before and after passage through the lungs. In prepulmonic blood taken from azygos vein or vena cava draining the marrow rich ribs, vertebrae, or pelvis, megakaryocytes are relatively numerous and very large, spherical or oval, commonly measuring 50 to 100 μm or more in diameter (Melamed et al, 1962, 1966). They have abundant, finely granular cytoplasm and a cytoplasmic membrane that is either sharply defined or ragged with adherent platelets. The nuclei are large and lobated, with well-defined chromatin structure. They contain multiple nucleoli, usually at least one in each lobe, but these are sometimes difficult to see in Papanicolaou stain. Within the nuclei, there are also multiple small areas of nuclear clearing, emphasized as characteristic by McGrew (1965) (Fig. 43-4).
Megakaryoblasts are somewhat smaller than mature megakaryocytes and have much less cytoplasm, a large round or bilobed nucleus with more delicate chromatin, and one or several nucleoli.
In postpulmonic pulmonary venous and peripheral blood, megakaryocytes are less numerous, sausage-shaped, and typically stripped of parts of the cytoplasm. Their nuclei are contracted or fragmented and usually dark-staining, though still lobated. Nuclear chromatin detail is often obscured (Fig. 43-5). Megakaryocytes have been described by us (Melamed et al, 1962, 1966) and others (Minot, 1922; Raker et al, 1960; Jackson, 1962; Hume et al, 1964) in peripheral blood of healthy individuals and patients with and without cancer. A megakaryocyte blood count is possible. Preliminary counts on normal individuals have shown marked individual variation without apparent hematologic abnormalities (Efrati and Rozenszagn, 1960; Melamed et al, 1966; Hansen and Pedersen, 1978). These studies also document that megakaryocytes normally enter the blood circulation and that platelet production, thought to occur exclusively in the bone marrow, may also take place in the lung as these large cells pass through the narrow pulmonary capillaries.
Myeloid, Lymphoid, and Other Blood Cells
Some immature myeloid and lymphoid cells may be similar in size to small epithelial cancer cells and mistaken for them.
The cytoplasmic granules in myeloid cells are indistinct with the Papanicolaou stain. Nuclei are rounded, reniform, or lobated. Nucleoli may be multiple and prominent, though they are rarely as large as those in many epithelial cancer cells. Plasma cells and their precursors may be recognized by the peripheral clumping of nuclear chromatin, often asymmetric positioning of the nucleus, and dark cytoplasmic staining with a paranuclear “hof” or clearing.
The cytoplasmic granules in myeloid cells are indistinct with the Papanicolaou stain. Nuclei are rounded, reniform, or lobated. Nucleoli may be multiple and prominent, though they are rarely as large as those in many epithelial cancer cells. Plasma cells and their precursors may be recognized by the peripheral clumping of nuclear chromatin, often asymmetric positioning of the nucleus, and dark cytoplasmic staining with a paranuclear “hof” or clearing.
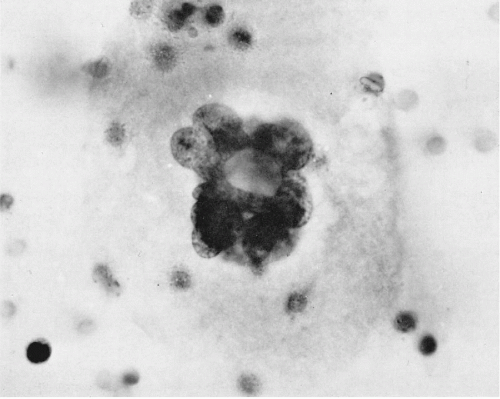 Figure 43-4 Megakaryocyte from the prepulmonic (azygos vein) blood. The cell is large with abundant granular cytoplasm and has a well-preserved, multilobulated nucleus with areas of clearing about many chromatin granules. (Seal’s Nuclepore sieve method; H&E; oil immersion.) (From Melamed MR, et al. The megakaryocyte blood count. Am J Med Sci 252: 301-309, 1966.) |
Large lymphocytes, monocytes, and mononuclear macrophages may measure as much as 20 μm in diameter. Usually, these cells have bland-appearing nuclei and, occasionally, small nucleoli. Poorly preserved cells of this type, or cells without specific characteristics, may be difficult to identify (Fig. 43-6). They can be mistaken for small cancer cells, particularly if they are degenerating and hyperchromatic. Such cells probably account for most of what are reported as unclassified “atypical” cells (Sandberg et al, 1959; Melamed et al, 1962; Nedelkoff et al, 1962).
 Figure 43-5 Megakaryocytes from post-pulmonic (antecubital vein) blood. The nuclei are contracted (or fragmented) and have lost most of their cytoplasm. The cell in A is elongated as if distorted by passage through a narrow capillary. (Seal’s Nuclepore sieve method; H&E; oil immersion.) (From Melamed MR, et al. The megakaryocyte blood count. Am J Med Sci, 252:301-309, 1966.) |
Endothelial and Other Cells
Endothelial cells can be dislodged by the needle as a specimen of blood is obtained. They may be present singly, in small sheets, or even as syncytia. They have delicate, transparent cytoplasm and regular, round or oval nuclei of 10 to 15 μm diameter with very delicate or finely punctate chromatin and at least one, sometimes prominent, nucleolus. It is worth deliberately preparing a few specimens that contain endothelial cells because their normal appearance is quite characteristic, though, like all other cells, there are circumstances in which they can become quite atypical
(Fig. 43-7). If necessary, their identity can be confirmed by immunostaining with Factor VIII or CD31.
(Fig. 43-7). If necessary, their identity can be confirmed by immunostaining with Factor VIII or CD31.
Epithelial (squamous) cells from the skin have been identified in blood specimens obtained by percutaneous venipuncture. Similarly, mesothelial cells have been found in blood samples obtained by transpericardial needle aspiration of the heart. These cells are morphologically identical to those observed in other types of preparations and should be readily recognized.
Among other unusual cells that have been identified on rare occasion are trophoblasts (Douglas et al, 1959), osteoclasts (Haemmerli and Straeuli, 1963), Gaucher cells, and mast cells (Alexander and Spriggs, 1960).
Significance of the Early Studies
In early studies, the likelihood of finding cancer cells in the blood of patients known to have cancer was variously reported from less than 1% to as much as 96.5% (McGrew et al, 1960; Christopherson, 1965; Goldblatt and Nadel, 1965; Griffiths, 1965; Griffiths et al, 1973; Nagy, 1965). In our own experience at that time, principally with patients having lung and breast cancer, cancer cells were found in the peripheral venous blood of less than 1% of the patients. Some of the extraordinary differences in the published results can be attributed to technical differences in the method of processing, the use of peripheral versus regional venous blood, differences in the number and volume of samples examined, and differences in the type of cancer and stage of the disease. The likelihood of finding cancer cells was greater in regional venous blood draining the cancer site than in peripheral venous or arterial blood, greater when more specimens or larger volumes of blood were examined (Gurian and West, 1963), greater in disseminated cancer, and probably greater when tumor masses were disturbed mechanically by manipulation during surgery or physical examination (Jonasson, 1961; Breslow et al, 1968; Roger et al, 1972; Turnbull et al, 1965). It is also worth noting that specimens of blood and perfusate from surgical specimens are not to be compared with blood samples drawn by venipuncture from the living patient. Selbach et al (1963) examined regional and inferior vena caval cadaveric blood from 18 patients who died of a variety of different carcinomas and found cancer cells in 50%. They attributed this to advanced disease rather than to any agonal or postmortem artifact. The study should be repeated with contemporary techniques.
The early studies of circulating cancer cells, though of relatively limited clinical value, provided a foundation for subsequent investigations using the new technologies described below. They identified the types of cells that may be observed, in small number, in the circulating blood and contributed to our understanding of megakaryocytic circulation and platelet formation (see above).
In retrospect, it is not surprising that the reported presence of circulating cancer cells and its relationship to metastasis and survival of patients with cancer was (and still is)
controversial. Engell (1959), in a follow-up of his earlier report, Potter and Malmgren (1958), and Song et al (1971), failed to demonstrate a convincing relationship between the presence or absence of circulating cancer cells, metastases and survival. On the other hand, Watne and associates (1960) did find a somewhat higher proportion of potentially curable patients among those who had no circulating cancer cells. Drye et al (1962) reported that the likelihood of tumor recurrence was greater in patients who had cancer cells in peripheral blood than in those who did not.
controversial. Engell (1959), in a follow-up of his earlier report, Potter and Malmgren (1958), and Song et al (1971), failed to demonstrate a convincing relationship between the presence or absence of circulating cancer cells, metastases and survival. On the other hand, Watne and associates (1960) did find a somewhat higher proportion of potentially curable patients among those who had no circulating cancer cells. Drye et al (1962) reported that the likelihood of tumor recurrence was greater in patients who had cancer cells in peripheral blood than in those who did not.
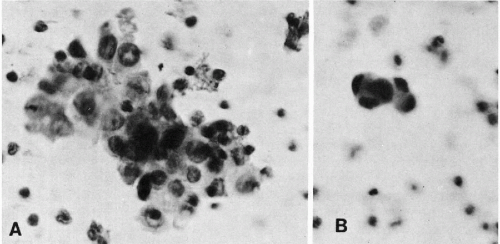
Roberts et al (1958, 1960, 1961a, 1961b, 1962), Cole et al (1961) and Jonasson et al (1961) described showers of tumor cells in the blood of patients immediately following surgical manipulation or trauma to their tumor and reported that those patients had a 2- to 5-year survival rate that was half that observed in patients without such findings during surgery. After 5 to 10 years follow-up, survival was unrelated to the finding of cancer cells in the blood, except when they occurred in showers, during or after operation (Roberts et al, 1967).
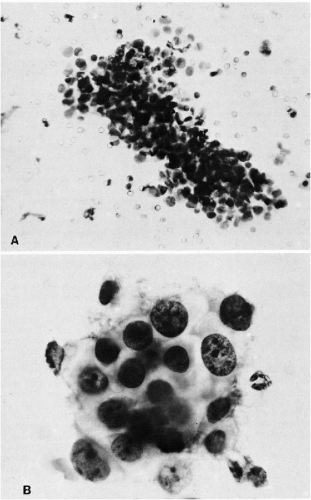
Some of the most rewarding early work came from studies of factors influencing metastases in experimental tumors of animals (Fig. 43-8A,B). With an experimental carcinoma of the cecum in rats, for example, the appearance of cancer cells in the blood was related to the development of metastases (Agostino et al, 1959; Cliffton and Agostino, 1961). Manipulation of a transplanted Walker 256 carcinosarcoma in the thigh of a rat was shown to cause showers of tumor




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree