Chronic Obstructive Pulmonary Disease
KEY CONCEPTS
![]() Chronic obstructive pulmonary disease (COPD) is a treatable and preventable disease characterized by progressive airflow limitation that is not fully reversible and is associated with an abnormal inflammatory response of the lungs to noxious particles or gases.
Chronic obstructive pulmonary disease (COPD) is a treatable and preventable disease characterized by progressive airflow limitation that is not fully reversible and is associated with an abnormal inflammatory response of the lungs to noxious particles or gases.
![]() COPD is historically described as either chronic bronchitis or emphysema. Chronic bronchitis is defined in clinical terms, whereas emphysema is defined in terms of anatomic pathology. Because most patients exhibit some features of each disease, the appropriate emphasis of COPD pathophysiology is on small airway disease and parenchymal damage that contributes to chronic airflow limitation.
COPD is historically described as either chronic bronchitis or emphysema. Chronic bronchitis is defined in clinical terms, whereas emphysema is defined in terms of anatomic pathology. Because most patients exhibit some features of each disease, the appropriate emphasis of COPD pathophysiology is on small airway disease and parenchymal damage that contributes to chronic airflow limitation.
![]() Mortality from COPD has increased steadily over the past three decades; it currently is the third leading cause of death in the United States.
Mortality from COPD has increased steadily over the past three decades; it currently is the third leading cause of death in the United States.
![]() The primary cause of COPD is cigarette smoking, implicated in 85% of diagnosed cases. Other risks include a genetic predisposition, environmental exposures (including occupational dusts and chemicals), and air pollution.
The primary cause of COPD is cigarette smoking, implicated in 85% of diagnosed cases. Other risks include a genetic predisposition, environmental exposures (including occupational dusts and chemicals), and air pollution.
![]() Smoking cessation, and avoidance of other known toxins causing COPD, are the only management strategies proven to slow the progression.
Smoking cessation, and avoidance of other known toxins causing COPD, are the only management strategies proven to slow the progression.
![]() Oxygen therapy has been shown to reduce mortality in selected patients with COPD. Oxygen therapy is indicated for patients with a resting PaO2 of less than 55 mm Hg or a PaO2 of less than 60 mm Hg and evidence of right-sided heart failure, polycythemia, or impaired neurologic function.
Oxygen therapy has been shown to reduce mortality in selected patients with COPD. Oxygen therapy is indicated for patients with a resting PaO2 of less than 55 mm Hg or a PaO2 of less than 60 mm Hg and evidence of right-sided heart failure, polycythemia, or impaired neurologic function.
![]() Bronchodilators represent the mainstay of drug therapy for COPD. Pharmacotherapy is used to relieve patient symptoms and improve quality of life. Guidelines recommend short-acting bronchodilators as initial therapy for patients with mild or intermittent symptoms.
Bronchodilators represent the mainstay of drug therapy for COPD. Pharmacotherapy is used to relieve patient symptoms and improve quality of life. Guidelines recommend short-acting bronchodilators as initial therapy for patients with mild or intermittent symptoms.
![]() For the patient who experiences chronic symptoms, long-acting bronchodilators are appropriate. Either a β2-agonist or an anticholinergic offers significant benefits. Combining long-acting bronchodilators is recommended if necessary, despite limited data.
For the patient who experiences chronic symptoms, long-acting bronchodilators are appropriate. Either a β2-agonist or an anticholinergic offers significant benefits. Combining long-acting bronchodilators is recommended if necessary, despite limited data.
![]() The role of inhaled corticosteroid therapy in COPD is controversial. International guidelines suggest that patients with severe COPD and frequent exacerbations may benefit from inhaled corticosteroids.
The role of inhaled corticosteroid therapy in COPD is controversial. International guidelines suggest that patients with severe COPD and frequent exacerbations may benefit from inhaled corticosteroids.
![]() Acute exacerbations of COPD have a significant impact on disease progression and mortality. Treatment of acute exacerbations includes intensification of bronchodilator therapy and a short course of systemic corticosteroids.
Acute exacerbations of COPD have a significant impact on disease progression and mortality. Treatment of acute exacerbations includes intensification of bronchodilator therapy and a short course of systemic corticosteroids.
![]() Antimicrobial therapy should be used during acute exacerbations of COPD if the patient exhibits at least two of the following: increased dyspnea, increased sputum volume, and increased sputum purulence.
Antimicrobial therapy should be used during acute exacerbations of COPD if the patient exhibits at least two of the following: increased dyspnea, increased sputum volume, and increased sputum purulence.
![]() Chronic obstructive pulmonary disease (COPD) is a common lung disease characterized by airflow limitation that is not fully reversible and is both chronic and progressive.1 COPD is preventable and treatable and causes significant extrapulmonary effects that contribute to disease severity in a subset of patients. The prevalence and mortality of COPD have increased substantially over the past 2 decades. Currently, COPD is the third leading cause of death in the United States, with 6/3% of adults reporting a physician diagnosis of COPD.2 By 2020, it is estimated that COPD will rank fifth in burden of disease and third as a cause of death throughout the world.
Chronic obstructive pulmonary disease (COPD) is a common lung disease characterized by airflow limitation that is not fully reversible and is both chronic and progressive.1 COPD is preventable and treatable and causes significant extrapulmonary effects that contribute to disease severity in a subset of patients. The prevalence and mortality of COPD have increased substantially over the past 2 decades. Currently, COPD is the third leading cause of death in the United States, with 6/3% of adults reporting a physician diagnosis of COPD.2 By 2020, it is estimated that COPD will rank fifth in burden of disease and third as a cause of death throughout the world.
Although national guidelines for management have been available for over 2 decades, questions have been raised concerning their quality and supporting evidence. In order to standardize the care of patients with COPD and present evidence-based recommendations, the National Heart, Lung, and Blood Institute (NHLBI) and the World Health Organization (WHO) launched the Global Initiative for Chronic Obstructive Lung Disease (GOLD) in 2001. This report was most recently revised in December 2013.1 The goals of the GOLD organization are to increase awareness of COPD and reduce morbidity and mortality associated with the disease. International guidelines have also been developed through a collaborative effort of the American College of Physicians (ACP), the American College of Chest Physicians (ACCP), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) and are widely available.3 These two guidelines are generally concordant in their recommendations.
COPD is differentiated from asthma in that the airflow limitation that is present is not fully reversible. In a subset of patients, it is fixed with minimal improvement in response to a bronchodilator or with optimal treatment. However, the natural course of the disease is quite variable among patients. The chronic and progressive nature of COPD is associated with an abnormal inflammatory response of the lungs to noxious particles or gases.1 Nonetheless, COPD is preventable and treatable. In recent years, there has been an increased appreciation for the impact of the systemic consequences of chronic inflammatory diseases, including COPD, and for the impact of comorbidities in individual patients that can complicate COPD management.
For many years, clinicians and researchers have exhibited a nihilistic attitude toward the value of treatments for COPD. This was based on the paucity of effective therapies, the destructive nature of the condition, and the fact that the common etiology is cigarette smoking, a modifiable health risk. There is now a renewed interest in evaluating the value of treatments and prevention based on the availability of new therapeutic options for pharmacotherapy and guidelines based on evidence. The international guidelines emphasize the terms preventable and treatable to support a positive approach to managing the patient with COPD. Support is also reflected in the availability of research funding to improve understanding about this disease and its management. This includes NHLBI funding of Specialized Centers of Clinically Oriented Research (SCCOR) programs in COPD that have an objective to promote multidisciplinary research on clinically relevant questions enabling basic science findings to be more rapidly applied to clinical problems.4
![]() The term COPD has historically been used to describe various pulmonary diseases with a fixed component of airflow limitation. The two principal conditions are chronic bronchitis and emphysema. Chronic bronchitis is associated with chronic or recurrent excessive mucus secretion into the bronchial tree with cough that is present on most days for at least 3 months of the year for at least 2 consecutive years in a patient in whom other causes of chronic cough have been excluded.3 While chronic bronchitis is defined in clinical terms, emphysema is defined in terms of anatomic pathology. Emphysema historically was defined on histologic examination at autopsy. Because this histologic definition is of limited clinical value, emphysema also has been defined as abnormal permanent enlargement of the airspaces distal to the terminal bronchioles accompanied by destruction of their walls, yet without obvious fibrosis.3
The term COPD has historically been used to describe various pulmonary diseases with a fixed component of airflow limitation. The two principal conditions are chronic bronchitis and emphysema. Chronic bronchitis is associated with chronic or recurrent excessive mucus secretion into the bronchial tree with cough that is present on most days for at least 3 months of the year for at least 2 consecutive years in a patient in whom other causes of chronic cough have been excluded.3 While chronic bronchitis is defined in clinical terms, emphysema is defined in terms of anatomic pathology. Emphysema historically was defined on histologic examination at autopsy. Because this histologic definition is of limited clinical value, emphysema also has been defined as abnormal permanent enlargement of the airspaces distal to the terminal bronchioles accompanied by destruction of their walls, yet without obvious fibrosis.3
Differentiating COPD as either chronic bronchitis or emphysema as descriptive subsets of COPD is no longer considered relevant. This is based on the observation that the majority of COPD is caused by a common risk factor (cigarette smoking) and most patients exhibit features of both chronic bronchitis and emphysema. Currently, emphasis is placed on the pathophysiologic features of small airways disease and parenchymal destruction as contributors to chronic airflow limitation. Most patients with COPD demonstrate features of both chronic bronchitis and emphysema. Chronic inflammation affects the integrity of the airways and causes damage and destruction of the parenchymal structures. The underlying problem is persistent exposure to noxious particles or gases that sustain the inflammatory response. The airways of both the lung and the parenchyma are susceptible to inflammation, and the result is the chronic airflow limitation that characterizes COPD (see Fig. 16-1).
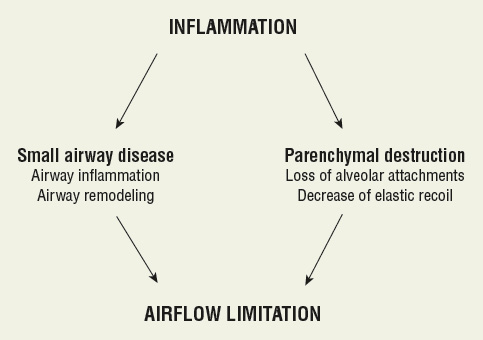
FIGURE 16-1 Mechanisms for developing chronic airflow limitation in COPD. (From reference 1.)
EPIDEMIOLOGY
The true prevalence of COPD is likely underreported in the United States. Data from the National Health Interview Survey in 2001 indicate that 12.1 million people over age 25 years have COPD.5 Over 9 million of these individuals have chronic bronchitis; the remaining number have emphysema or a combination of both diseases. According to national surveys, the true prevalence of people with symptoms of chronic airflow obstruction may exceed 24 million.6 The burden may be even greater because more than one third of adults in the United States reported respiratory complaints compatible with symptomatic COPD in some surveys.7
![]() COPD is the fourth leading cause of death in the United States, exceeded only by cancer, heart disease, and cerebrovascular accidents. In 2005, COPD accounted for 126,005 deaths in the United States, representing 1 in every 20 deaths. Deaths attributed to COPD increased 8% from 2000 to 2005.8 It is the only leading cause of death to increase over the past 30 years and is projected to be the third leading cause by 2020.9 Overall, the mortality rate is higher in males; however, the female death rate has doubled over the past 25 years, and the number of female deaths exceeded male deaths in each year since 2000. The mortality rate is higher in whites compared with that in blacks.9
COPD is the fourth leading cause of death in the United States, exceeded only by cancer, heart disease, and cerebrovascular accidents. In 2005, COPD accounted for 126,005 deaths in the United States, representing 1 in every 20 deaths. Deaths attributed to COPD increased 8% from 2000 to 2005.8 It is the only leading cause of death to increase over the past 30 years and is projected to be the third leading cause by 2020.9 Overall, the mortality rate is higher in males; however, the female death rate has doubled over the past 25 years, and the number of female deaths exceeded male deaths in each year since 2000. The mortality rate is higher in whites compared with that in blacks.9
Cigarette smoking is the primary cause of COPD and, although the prevalence of cigarette smoking has declined compared with 1965, approximately 25% of individuals in the United States currently smoke. The trend of increasing COPD mortality likely reflects the long latency period between smoking exposure and complications associated with COPD.
The mortality of COPD is significant; however, morbidity associated with the disease also has a significant impact on patients, their families, and the healthcare system. COPD represents the second leading cause of disability in the United States. In the last 20 years, COPD has been responsible for nearly 50 million hospital visits nationwide.10 In recent years, a diagnosis of COPD accounts for over 15 million physician office visits, 1.5 million emergency room visits, and 700,000 hospitalizations annually. A survey by the American Lung Association revealed that among COPD patients, 51% reported that their condition limits their ability to work, 70% were limited in normal physical activity, 56% were limited in performing household chores, and 50% reported that sleep was affected adversely.11
The economic impact of COPD continues to increase as well. It was estimated at $23 billion in 2000 and rose to $37.2 billion in 2004, including $20.9 billion in direct costs and $16.3 billion in indirect morbidity and mortality costs.9,12 By 2020, COPD will be the fifth most burdensome disease, as measured by disability-adjusted life years lost due to illness. The cost of care for COPD patients is high compared with that for patients without the disease. There is a relationship among the severity of COPD, resources consumed, and the costs of care.13
ETIOLOGY
![]() Cigarette smoking is the most common risk factor and accounts for 85% to 90% of cases of COPD.1 Components of tobacco smoke activate inflammatory cells, which produce and release the inflammatory mediators characteristic of COPD. Smokers are 12 to 13 times more likely to die from COPD than nonsmokers.14 Although the risk is lower in pipe and cigar smokers, it is still higher than in nonsmokers. Age of starting, total pack-years, and current smoking status are predictive of COPD mortality.
Cigarette smoking is the most common risk factor and accounts for 85% to 90% of cases of COPD.1 Components of tobacco smoke activate inflammatory cells, which produce and release the inflammatory mediators characteristic of COPD. Smokers are 12 to 13 times more likely to die from COPD than nonsmokers.14 Although the risk is lower in pipe and cigar smokers, it is still higher than in nonsmokers. Age of starting, total pack-years, and current smoking status are predictive of COPD mortality.
However, only 15% to 20% of all smokers go on to develop COPD, and not all smokers who have equivalent smoking histories develop the same degree of pulmonary impairment, suggesting that other host and environmental factors contribute to the degree of lung dysfunction. Nevertheless, the rate of loss of lung function is determined primarily by smoking status and history.3 Children and spouses of smokers have increased risk of developing significant pulmonary dysfunction through passive smoking, also known as environmental tobacco smoke or secondhand smoke.
In addition to cigarette smoking, COPD is attributed to a combination of risk factors that results in lung injury and tissue destruction. Risk factors can be divided into host factors and environmental factors (Table 16-1), and, commonly, the interaction between these risks leads to expression of the disease. Host factors, such as genetic predisposition, may not be modifiable but are important for identifying patients at high risk of developing the disease.
TABLE 16-1 Risk Factors for Development of Chronic Obstructive Pulmonary Disease (COPD)
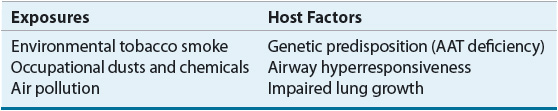
Environmental factors, such as tobacco smoke and occupational dust and chemicals, are modifiable factors that, if avoided, may reduce the risk of disease development. Environmental exposures associated with COPD are particles that are inhaled by the individual, which result in inflammation and cell injury. Exposure to multiple environmental toxins increases the risk of COPD. Thus, the total burden of inhaled particles (e.g., cigarette smoke as well as occupational and environmental particles and pollutants) can play a significant role in the development of COPD. In such cases, it is helpful to assess an individual’s total burden of inhaled particles. For example, an individual who smokes and works in a textile factory has a higher total burden of inhaled particles than an individual who smokes and has no occupational exposure.
In nonindustrialized countries, occupational exposures may be a more common risk than cigarette smoking. These exposures include dust and chemicals such as vapors, irritants, and fumes. Reduced lung function and deaths from COPD are higher for individuals who work in gold and coal mining, in the glass or ceramic industries with exposure to silica dust, and in jobs that expose them to cotton dust or grain dust, toluene diisocyanate, or asbestos. Other occupational risk factors include chronic exposure to open cooking or heating fires.
It is unclear whether air pollution alone is a significant risk factor for the development of COPD in smokers and nonsmokers with normal lung function. However, in individuals with existing pulmonary dysfunction, significant air pollution worsens symptoms. As evidence for this, emergency department visits are increased during higher-intensity periods of air pollution.
Individuals exposed to the same environmental risk factors do not have the same chance of developing COPD, suggesting that host factors play an important role in pathogenesis.1,3 While many not-yet-identified genes may influence the risk of developing COPD, the best documented genetic factor is a hereditary deficiency of α1-antitrypsin (AAT). AAT-associated emphysema is an example of a pure genetic disorder inherited in an autosomal recessive pattern. Inheritance is sometimes described as autosomal codominant by some researchers, because heterozygotes can also have decreased concentrations of AAT enzyme.15 The consequences of AAT deficiency are discussed in the following section as protease–antiprotease imbalance. True AAT deficiency accounts for less than 1% of COPD cases.3
AAT is a 42 kDa plasma protein that is synthesized in hepatocytes. A primary role of AAT is to protect cells, especially those in the lung, from destruction by elastase released by neutrophils. In fact, AAT may be responsible for 90% of the inhibition of this destructive enzyme.16 In individuals with the most common allele (M), plasma levels of AAT are approximately 20 to 50 μmol (100 to 350 mg/dL). The protective effect of AAT in the lungs is significantly diminished when plasma levels are less than 11 μmol (80 mg/dL).16 AAT is an acute-phase reactant, and the serum concentration can be quite variable.
Several types of AAT deficiency have been identified and are due to mutations in the AAT gene. Two main gene variants, S and Z, have been identified. For patients who are homozygous with the S variant, AAT levels are at least 60% of those of normal individuals. These patients usually do not have an increased risk of COPD compared with normal individuals. Patients with homozygous Z deficiency (ZZ) represent 95% of clinical cases15 and have AAT levels that are 10% of those of normal individuals, while patients with heterozygous Z variant (SZ) have levels closer to 40% of those of normal individuals. Homozygous Z patients have a higher risk of developing COPD compared with heterozygous Z patients. A history of cigarette smoking increases this risk. A small number of patients have a null phenotype and are at high risk for developing emphysema because they produce virtually no AAT.
Patients with AAT deficiency develop COPD at an early age (20 to 50 years) primarily owing to an accelerated decline in lung function. Compared with an average annual decline in forced expiratory volume in 1 second (FEV1) of 25 mL/y in healthy nonsmokers, patients with homozygous Z deficiency have been reported to have declines of 54 mL/y for nonsmokers and 108 mL/y for current smokers. Effective diagnosis is dependent on clinical suspicion, diagnostic testing of serum concentrations, and genotype confirmation.15 Patients developing COPD at an early age or those with a strong family history of COPD should be screened for AAT deficiency. If the concentration is low, genotype testing (DNA) should be performed.
Other genes have been implicated with increased risk of developing COPD, including chromosome 2q, transforming growth factor β1, microsomal epoxide hydrolase 1, and tumor necrosis factor-α (TNF-α). However, there are no definite conclusions about an association other than AAT. One genetic factor that may reduce the risk of developing COPD is a polymorphism in the gene encoding for matrix metalloproteinase 12 (MMP12). A cohort of smokers with the polymorphism exhibited a lower risk for developing COPD (0.63).17
Two additional host factors that may influence the risk of COPD include airway hyperresponsiveness and lung growth. Individuals with airway hyperresponsiveness to various inhaled particles may have an accelerated decline in lung function compared with those without airway hyperresponsiveness. Additionally, individuals who do not attain maximal lung growth owing to low-birth-weight, prematurity at birth, or childhood illnesses may be at risk for COPD in the future.1
PATHOPHYSIOLOGY
COPD is characterized by chronic inflammatory changes that lead to destructive changes and the development of chronic airflow limitation. The inflammatory process is widespread and not only involves the airways but also extends to the pulmonary vasculature and lung parenchyma. The inflammation of COPD is often referred to as neutrophilic in nature, but macrophages and CD8+ lymphocytes also play major roles.18–20 The inflammatory cells release a variety of chemical mediators, of which TNF-α, interleukin 8 (IL-8), and leukotriene B4 (LTB4) play major roles.1,21 The actions of these cells and mediators are complementary and redundant, leading to the widespread destructive changes. The stimulus for activation of inflammatory cells and mediators is an exposure to noxious particles and gas through inhalation. The most common etiologic factor is exposure to environmental tobacco smoke, although other chronic inhalational exposures can lead to similar inflammatory changes.
Other processes that have been proposed to play a major role in the pathogenesis of COPD include oxidative stress and an imbalance between aggressive and protective defense systems in the lungs (proteases and antiproteases).18 These processes may be the result of ongoing inflammation or occur as a result of environmental pressures and exposures (Fig. 16-2).
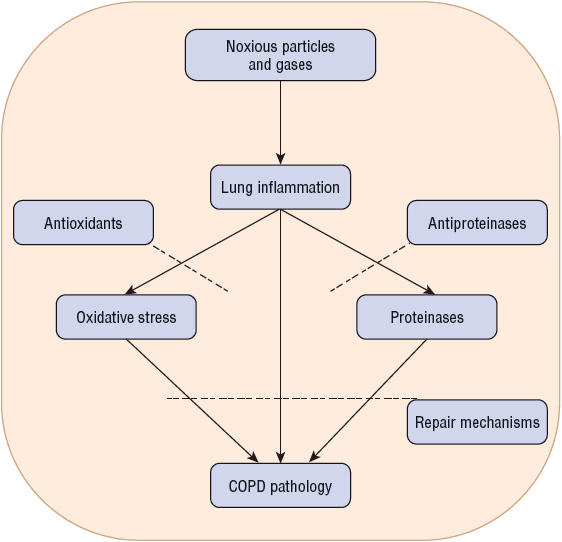
FIGURE 16-2 Pathogenesis of COPD.
An altered interaction between oxidants and antioxidants present in the airways is responsible for the increased oxidative stress present in COPD. Increases in markers (e.g., hydrogen peroxide and nitric oxide) of oxidants are seen in the epithelial lining fluid.1 The increased oxidants generated by cigarette smoke react with and damage various proteins and lipids, leading to cell and tissue damage. Oxidants also promote inflammation directly and exacerbate the protease–antiprotease imbalance by inhibiting antiprotease activity.
The consequences of an imbalance between proteases and antiproteases in the lungs were described over 40 years ago when the hereditary deficiency of the protective antiprotease AAT was discovered to result in an increased risk of developing emphysema prematurely. This enzyme (AAT) is responsible for inhibiting several protease enzymes, including neutrophil elastase. In the presence of unopposed activity, elastase attacks elastin, a major component of alveolar walls.1
In the inherited form of emphysema, there is an absolute deficiency of AAT. In cigarette smoking–associated emphysema, the imbalance is likely associated with increased protease activity or reduced activity of antiproteases. Activated inflammatory cells release several proteases other than AAT, including cathepsins and metalloproteinases (MMPs). In addition, oxidative stress reduces antiprotease (or protective) activity.
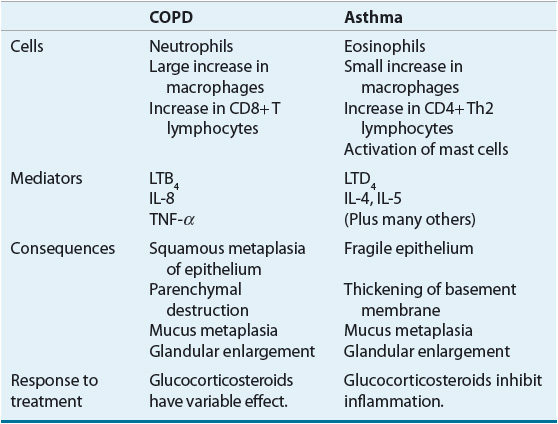
It is helpful to differentiate inflammation occurring in COPD from that present in asthma because the response to antiinflammatory therapy differs. The inflammatory cells that predominate differ between the two conditions, with neutrophils playing a major role in COPD and eosinophils and mast cells in asthma. Mediators of inflammation also differ leukotriene B4 (LTB4), interleukin 8 (IL-8), and tumor necrosis factor alpha (TNF-α) predominating in COPD, compared with leukotriene D4 (LTD4), interleukin 4 (IL-4), and interleukin 5 (IL-5) among the numerous mediators modulating inflammation in asthma.1 Characteristics of inflammation for the two diseases are summarized in Table 16-2.
TABLE 16-2 Features of Inflammation in COPD Compared with Asthma
Pathologic changes of COPD are widespread, affecting large and small airways, lung parenchyma, and the pulmonary vasculature.1 An inflammatory exudate is often present that leads to an increase in the number and size of goblet cells and mucus glands. Mucus secretion is increased, and ciliary motility is impaired. There is also a thickening of smooth muscle and connective tissue in the airways. Inflammation is present in central and peripheral airways. The chronic inflammation results in a repeated injury and repair process that leads to scarring and fibrosis. Diffuse airway narrowing is present and is more prominent in smaller peripheral airways. The decrease in FEV1 is attributed to the presence of inflammation in the airways, while the blood gas abnormalities result from impaired gas transfer due to parenchymal damage.
Parenchymal changes affect the gas-exchanging units of the lungs, including the alveoli and pulmonary capillaries. The distribution of destructive changes varies depending on the etiology. Most commonly, smoking-related disease results in centrilobular emphysema that primarily affects respiratory bronchioles. Panlobular emphysema is seen in AAT deficiency and extends to the alveolar ducts and sacs.
The vascular changes of COPD include a thickening of pulmonary vessels and often are present early in the disease. Increased pulmonary pressures early in the disease are due to hypoxic vasoconstriction of pulmonary arteries. If persistent, the presence of chronic inflammation may lead to endothelial dysfunction of the pulmonary arteries. Later, structural changes lead to an increase in pulmonary pressures, especially during exercise. In severe COPD, secondary pulmonary hypertension leads to the development of right-sided heart failure.
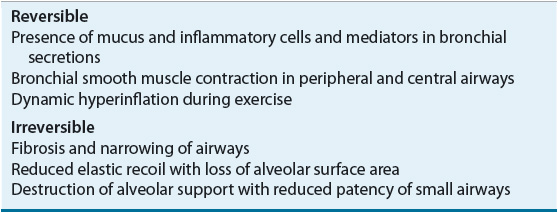
Mucus hypersecretion is present early in the course of the disease and is associated with an increased number and size of mucus-producing cells. The presence of chronic inflammation perpetuates the process, although the resulting airflow obstruction and chronic airflow limitation may be reversible or irreversible. The various causes of airflow obstruction are summarized in Table 16-3.
TABLE 16-3 Etiology of Airflow Limitation in COPD
Thoracic overinflation is a relevant feature in the pathophysiology of COPD, because it is a central factor in causing dyspnea. Chronic airflow obstruction leads to air trapping, resulting in thoracic hyperinflation that can be detected on chest radiograph. This problem results in several dynamic changes in the chest, including flattening of diaphragmatic muscles. Under normal circumstances, the diaphragms are dome-shaped muscles tethered at the base of the lungs. When the diaphragm contracts, the muscle becomes shorter and flatter, which creates the negative inspiratory force through which air flows into the lung during inspiration. In the presence of thoracic hyperinflation, the diaphragmatic muscle is placed at a disadvantage and is a less efficient muscle of ventilation. The increased work required by diaphragmatic contractions predisposes the patient to muscle fatigue, especially during periods of exacerbations.
The other consequence of thoracic hyperinflation is a change in lung volumes. For patients with COPD who exhibit thoracic hyperinflation, there is an increase in the functional residual capacity (FRC), which is the amount of air left in the lung after exhalation at rest. Therefore, these patients are breathing at higher lung volumes, which perturbs gas exchange. In addition, the increased FRC limits the inspiratory reserve capacity, which is the amount of air that the patient can inhale to fill the lungs. The increased FRC also limits the duration of inhalation time, and this has been associated with an increase in dyspnea complaints by patients.22 Drug therapy for COPD, especially bronchodilators, can reduce thoracic hyperinflation by reducing airflow obstruction. This may partially explain the improvement in symptoms reported by patients with COPD despite minimal improvements in lung function with drug therapy.
Airflow limitation is assessed through spirometry, which represents the “gold standard” for diagnosing and monitoring COPD. The hallmark of COPD is a reduction in the ratio of FEV1 to forced vital capacity (FVC) to less than 70%.1,3 The FEV1 generally is reduced, except in very mild disease, and the rate of FEV1 decline is greater in COPD patients compared with that in normal subjects.
The impact of the numerous pathologic changes in the lung perturbs the normal gas-exchange and protective functions of the lung. Ultimately, these are exhibited through the common symptoms of COPD, including dyspnea and a chronic cough productive of sputum. As the disease progresses, abnormalities in gas exchange lead to hypoxemia and/or hypercapnia, although there often is not a strong relationship between pulmonary function and arterial blood gas (ABG) results.
Significant changes in ABGs usually are not present until the FEV1 is less than 1 L.1 In these patients, hypoxemia and hypercapnia can become chronic problems. Initially, when hypoxemia is present, it usually is associated with exercise. However, as the disease progresses, hypoxemia at rest develops. Patients with severe COPD can have a low arterial oxygen tension (pressure exerted by oxygen gas in arterial blood [PaO2] = 45 to 60 mm Hg) and an elevated arterial carbon dioxide tension (pressure exerted by carbon dioxide gas in arterial blood [PaCO2] = 50 to 60 mm Hg). The hypoxemia is attributed to hypoventilation ![]() of lung tissue relative to perfusion
of lung tissue relative to perfusion ![]() of the area. This low
of the area. This low ![]() ratio will progress over a period of several years, resulting in a consistent decline in the PaO2. Some COPD patients lose the ability to increase the rate or depth of respiration in response to persistent hypoxemia. Although this is not completely understood, the decreased ventilatory drive may be due to abnormal peripheral or central respiratory receptor responses. This relative hypoventilation subsequently leads to hypercapnia. In this case, the central respiratory response to a chronically increased PaCO2 can be blunted. These changes in PaO2 and PaCO2 are subtle and progress over a period of many years. As a result, the pH usually is nearly normal because the kidneys compensate by retaining bicarbonate. If acute respiratory distress develops, such as might be seen in pneumonia or a COPD exacerbation with impending respiratory failure, the PaCO2 may rise sharply, and the patient presents with an uncompensated respiratory acidosis.
ratio will progress over a period of several years, resulting in a consistent decline in the PaO2. Some COPD patients lose the ability to increase the rate or depth of respiration in response to persistent hypoxemia. Although this is not completely understood, the decreased ventilatory drive may be due to abnormal peripheral or central respiratory receptor responses. This relative hypoventilation subsequently leads to hypercapnia. In this case, the central respiratory response to a chronically increased PaCO2 can be blunted. These changes in PaO2 and PaCO2 are subtle and progress over a period of many years. As a result, the pH usually is nearly normal because the kidneys compensate by retaining bicarbonate. If acute respiratory distress develops, such as might be seen in pneumonia or a COPD exacerbation with impending respiratory failure, the PaCO2 may rise sharply, and the patient presents with an uncompensated respiratory acidosis.
The consequences of long-standing COPD and chronic hypoxemia include the development of secondary pulmonary hypertension that progresses slowly if appropriate treatment of COPD is not initiated. Pulmonary hypertension is the most common cardiovascular complication of COPD and can result in cor pulmonale, or right-sided heart failure.23
The elevated pulmonary artery pressures are attributed to vasoconstriction (in response to chronic hypoxemia), vascular remodeling, and loss of pulmonary capillary beds. If elevated pulmonary pressures are sustained, cor pulmonale can develop, characterized by hypertrophy of the right ventricle in response to increases in pulmonary vascular resistance.
The risks of cor pulmonale include venous stasis with the potential for thrombosis and pulmonary embolism. Another important systemic consequence of COPD is a loss of skeletal muscle mass and general decline in the overall health status.
While airway inflammation is prominent for patients with COPD, there is also evidence of systemic inflammation.24 A consequence is widespread skeletal muscle dysfunction, especially in the leg muscles involved with ambulation.25 The systemic manifestations can have devastating effects on overall health status and comorbidities. These include cardiovascular events associated with ischemia, cachexia, osteoporosis, anemia, and muscle wasting. There is some interest in the role of measuring C-reactive protein as a parameter to assess systemic inflammation and its impact on COPD severity; however, it is premature to recommend this strategy currently.26
PATHOPHYSIOLOGY OF EXACERBATION
The natural history of COPD is characterized by recurrent exacerbations associated with increased symptoms and a decline in overall health status. An exacerbation is defined as a change in the patient’s baseline symptoms (dyspnea, cough, or sputum production) beyond day-to-day variability sufficient to warrant a change in management.1,3 Exacerbations have a significant impact on the natural course of COPD and occur more frequently for patients with more severe chronic disease. Because many patients experience chronic symptoms, the diagnosis of an exacerbation is based, in part, on subjective measures and clinical judgment. Repeated exacerbations, especially those requiring hospitalization, are associated with an increased mortality risk.
There are limited data about pathology during exacerbations owing to the nature of the disease and the condition of patients. However, inflammatory mediators including neutrophils and eosinophils are increased in the sputum. Chronic airflow limitation is a feature of COPD and may not change remarkably even during an exacerbation.1 The lung hyperinflation present in chronic COPD is worsened during an exacerbation, which contributes to worsening dyspnea and poor gas exchange.
The primary physiologic change is often a worsening of ABG results due to poor gas exchange and increased muscle fatigue. For a patient experiencing a severe exacerbation, profound hypoxemia and hypercapnia can be accompanied by respiratory acidosis and respiratory failure.
CLINICAL PRESENTATION
CLINICAL PRESENTATION
The diagnosis of COPD is made based on the patient’s symptoms, including cough, sputum production, and dyspnea, and a history of exposure to risk factors such as tobacco smoke and occupational exposures. Patients may have these symptoms for several years before dyspnea develops and often will not seek medical attention until dyspnea is significant. A diagnosis of COPD should be considered for any patient, age 40 years or older, with persistent or progressive dyspnea, with chronic cough productive of sputum, and who exhibits an unusual or abnormal decline in activity, especially in the presence of positive cigarette smoke exposure. In addition, the presence of genetic factors, including AAT deficiency, and occupational exposures should be evaluated because approximately 15% of patients with COPD do not have a history of cigarette smoking.
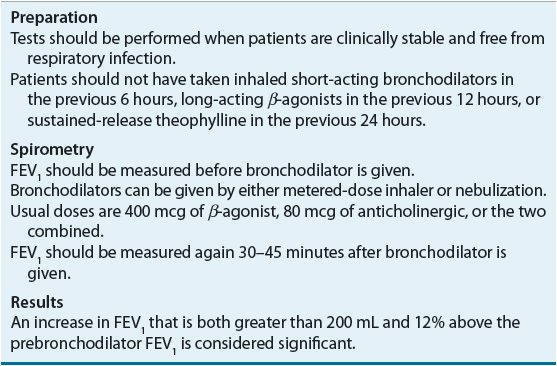
The presence of airflow limitation should be confirmed with spirometry. Spirometry represents a comprehensive assessment of lung volumes and capacities. The hallmark of COPD is an FEV1:FVC ratio of less than 70%, which indicates airway obstruction, and a postbronchodilator FEV1 of less than 80% of predicted confirms the presence of airflow limitation that is not fully reversible.1 There is an increased awareness that the use of a fixed ratio of less than 70% may be problematic because normal aging may affect this result; however, it continues to be the current standard. An improvement in FEV1 of less than 12% following inhalation of a rapid-acting bronchodilator is considered to be evidence of irreversible airflow obstruction. Reversibility of airflow limitation is measured by a bronchodilator challenge, which is described in Table 16-4. The use of peak expiratory flow measurements is not adequate for the diagnosis of COPD owing to low specificity and the high degree of effort dependence; however, a low peak expiratory flow is consistent with COPD. A comprehensive discussion about spirometry can be found in Chapter 15.
TABLE 16-4 Procedures for Reversibility Testing
Spirometry combined with a physical examination improves the diagnostic accuracy of COPD.7 Spirometry also is useful to determine the severity of airflow limitation. Patients with all levels of severity of COPD exhibit the hallmark finding of airflow obstruction, that is, a reduction in the FEV1:FVC ratio to less than 70%. FVC is the total amount of air exhaled after a maximal inhalation. Currently, the GOLD consensus guidelines suggest a four-grade classification of airflow limitation (see Table 16-5). Patients in GOLD 3 or 4 have the most significant airflow limitation and are at the highest risk for future exacerbations, while patients in GOLD 1 and 2 have less airflow limitation and are at lower risk for exacerbations.
TABLE 16-5 Classification of Severity of Airflow Obstruction (Based on Postbronchodilator FEV1)

Dyspnea is typically the most troublesome complaint for the patient with COPD and often is the stimulus for the patient seeking medical attention. It can impair exercise performance and functional capacity and is frequently associated with depression and anxiety. Together, these have a significant effect on health-related quality of life.22 As a subjective symptom, dyspnea is often difficult for the clinician to assess. Various tools are available to evaluate the severity of dyspnea. The modified Medical Research Council (mMRC) scale is commonly employed and categorizes dyspnea grades from 0 to 4 (see Table 16-6).27 The effect of COPD on overall well-being can be assessed using the COPD Assessment Test (CAT), which includes eight statements about symptoms and activities. The patient scores each statement on a scale of 0 to 5 and the impact of COPD is assessed by the cumulative score (see Table 16-7).28
TABLE 16-6 Modified Medical Research Council (MRC) Dyspnea Scale
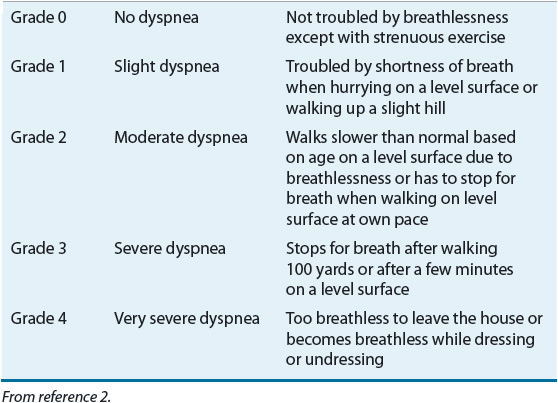
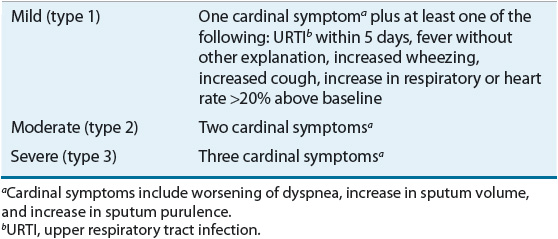
TABLE 16-7 Staging Acute Exacerbations of COPDa
Previously, guidelines have defined disease severity solely by spirometry. Observations that patients with similar spirometric parameters exhibit variations in symptom severity and risk of adverse health events, such as exacerbations, have led to a revision in severity classification. In order to incorporate multiple factors that contribute to disease risk, the revised GOLD consensus guidelines recommend that three separate parameters be assessed when classifying disease severity. Parameters include an assessment of airflow limitation by spirometry, measurement of symptom severity, and an assessment of exacerbation frequency. Symptom assessment should be measured using either CAT or mMRC. Frequency of exacerbations can be assessed either by predicted risk of future exacerbations based on classification of airflow limitation or through a review of exacerbation history for the past 12 months. Patients with at least two exacerbations in the last 12 months would be considered high risk for future exacerbations. If both methods of exacerbation risk are assessed, the method with the highest risk result should be used to classify the patient (see Fig. 16-3).
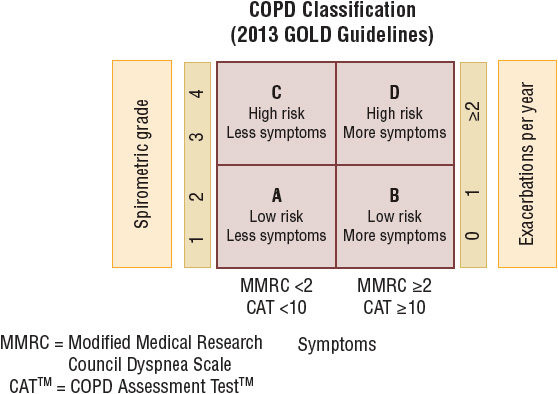
FIGURE 16-3 Groups of COPD classification.
While a physical examination is appropriate in the diagnosis and assessment of COPD, most patients who present in the milder stages of COPD will have a normal physical examination. In later stages of the disease, when airflow limitation is severe, patients may have cyanosis of mucosal membranes, development of “barrel chest” due to hyperinflation of the lungs, an increased respiratory rate and shallow breathing, and changes in breathing mechanics such as pursing of the lips to help with expiration or use of accessory respiratory muscles.
Classification Based on Severity
In 2011, the GOLD guidelines included a modified system for classifying COPD based on severity. As discussed above, the new system is based on numerous factors that have a significant impact on the patient, including the degree of airflow obstruction, the frequency and severity of symptoms, and the frequency of exacerbations (see Fig. 16-3). A patient can first be classified according to the severity of airflow obstruction into grades ranging from 1 to 4 (Table 16-5). Then the patient is placed into a group (A, B, C, or D) based on the impact of symptoms and the risk for future exacerbations. The extent of symptoms is assessed using a validated symptom assessment tool (e.g., the mMRC or the CAT). Finally, the risk for an exacerbation is based on previous exacerbations. A patient is categorized based on a history of less than two annual exacerbations, or two or more. This new classification system by group provides an appropriate emphasis for each of the parameters included (see Fig. 16-3). Another advantage is that classifying patients according to these groups informs treatment decisions.
Prognosis
For the patient with COPD, the combination of impaired lung function and recurrent exacerbations promotes a clinical scenario characterized by dyspnea, reduced exercise tolerance and physical activity, and deconditioning. These factors lead to disease progression, poor quality of life, possible disability, and premature mortality.29 COPD is ultimately a fatal disease if it progresses and advanced directives and end-of-life care options are appropriate to consider. The primary causes of death of patients with COPD include respiratory failure, cardiovascular events or diseases, and lung cancer.30
The FEV1 is the most important prognostic indicator for a patient with COPD. The average rate of decline of FEV1 is the most useful objective measure to assess the course of COPD. The average rate of decline in FEV1 for healthy, nonsmoking patients owing to age alone is 25 to 30 mL/y. The rate of decline for smokers is steeper, especially for heavy smokers compared with light smokers. The decline in pulmonary function is a steady curvilinear path. The more severely diminished the FEV1 at diagnosis, the steeper is the rate of decline. Greater numbers of years of smoking and number of cigarettes smoked also correlate with a steeper decline in pulmonary function.27 Conversely, the rate of decline of blood gases has not been shown to be a useful parameter to assess progression of the disease. Patients with COPD should have spirometry performed at least annually to assess disease progression.31
The survival rate of patients with COPD is highly correlated to the initial level of impairment in the FEV1 and age. Other less important factors include degree of reversibility with bronchodilators, resting pulse, perceived physical disability, diffusing capacity for carbon monoxide (DLCO), cor pulmonale, and blood gas abnormalities. A rapid decline in pulmonary function tests indicates a poor prognosis. Median survival is approximately 10 years when the FEV1 is 1.4 L, 4 years when the FEV1 is 1 L, and about 2 years when the FEV1 is 0.5 L.
While ABG measurements are important, they do not carry the prognostic value of pulmonary function tests. Measurement of ABGs is more useful for patients with severe disease and is recommended for all patients with an FEV1 of less than 40% of predicted or those with signs of respiratory failure or right-sided heart failure.1
Asthma is usually differentiated from COPD based on the patient’s medical history, risk factors, and improvements on postbronchodilator spirometry; however, in some cases, asthma patients exhibit COPD-like features and COPD patients exhibit asthma-like features. It is also possible for the two conditions to coexist.
CLINICAL PRESENTATION OF COPD EXACERBATION
Because of the subjective nature of defining an exacerbation of COPD, the criteria used among clinicians vary widely; however, most rely on a change in one or more of the following clinical findings: worsening symptoms of dyspnea, increase in sputum volume, or increase in sputum purulence. Acute exacerbations have a significant impact of the economics of treating COPD as well, estimated at 35% to 45% of the total costs of the disease in some settings.32
A widely accepted definition of an exacerbation is that it is an event in the natural course of COPD that is characterized by a worsening in baseline dyspnea, cough, and/or sputum that is beyond the normal day-to-day variation, is acute in onset, and may warrant a change in regular medication. With an exacerbation, patients using rapid-acting bronchodilators may report an increase in the frequency of use. Exacerbations are commonly staged as mild, moderate, or severe according to the criteria summarized in Table 16-7.33
An important complication of a severe exacerbation is acute respiratory failure. In the emergency department or hospital, an ABG usually is obtained to assess the severity of an exacerbation. The diagnosis of acute respiratory failure in COPD is made based on an acute change in the ABGs. Defining acute respiratory failure as a PaO2 of less than 50 mm Hg or a PaCO2 of greater than 50 mm Hg often may be incorrect and inadequate because these values may not represent a significant change from a patient’s baseline values. A more precise definition is an acute drop in PaO2 of 10 to 15 mm Hg or any acute increase in PacO2 that decreases the serum pH to 7.3 or less. Additional acute clinical manifestations of respiratory failure include restlessness, confusion, tachycardia, diaphoresis, cyanosis, hypotension, irregular breathing, miosis, and unconsciousness.
Prognosis
COPD exacerbations are associated with significant morbidity and mortality. While mild exacerbations may be managed at home, mortality rates are higher for patients admitted to the hospital. In one study of patients hospitalized with COPD exacerbations, in-hospital mortality was 6% to 8%.34 Many patients experiencing an exacerbation do not have a return to their baseline clinical status for several weeks, significantly affecting their quality of life. Additionally, as many as half the patients originally hospitalized for an exacerbation are readmitted within 6 months.35
There is good evidence that acute exacerbations of COPD have a tremendous impact on disease progression and ultimate mortality. For exacerbations requiring hospitalizations, mortality rates range from 22% to 43% after 1 year, and 36% to 49% in 2 years.34,36,37
TREATMENT
Chronic Obstructive Pulmonary Disease
Desired Outcome
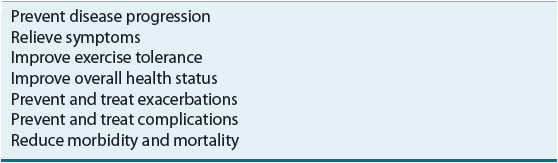
Given the nature of COPD, a major focus in healthcare should be on prevention. However, for patients with a diagnosis of COPD, the primary goal is to prevent or minimize progression. Specific goals of management are listed in Table 16-8. The primary goal of pharmacotherapy has been relief of symptoms, including dyspnea. However, more recently there has been increased interest in the value of therapeutic interventions that reduce exacerbation frequency and severity, as well as reduce mortality. In fact, a reduction in exacerbation frequency is an important outcome measure to consider when evaluating the role and benefit of individual chronic therapies used in COPD management.
TABLE 16-8 Goals of COPD Management
Optimally, these goals can be accomplished with minimal risks or side effects. The therapy of the patient with COPD is multifaceted and includes pharmacologic and nonpharmacologic strategies. Appropriate measures of effectiveness of the management plan include continued smoking cessation, symptom improvement, reduction in FEV1 decline, reduction in the number of exacerbations, improvements in physical and psychological well-being, and reduction in mortality, hospitalizations, and days lost from work.
CLINICAL PRESENTATION Features of COPD Exacerbation
Unfortunately, most treatments for COPD have not been shown to improve survival or to slow the progressive decline in lung function. However, many therapies do improve pulmonary function and quality of life and reduce exacerbations and duration of hospitalization. Several disease-specific quality-of-life measures are available to assess the overall efficacies of therapies for COPD, including the CAT, Chronic Respiratory Questionnaire (CRQ), and the St. George’s Respiratory Questionnaire (SGRQ). These questionnaires measure the impact of various therapies on such disease variables as severity of dyspnea and level of activity. They do not measure impact of therapies on survival. While early studies of COPD therapies focused primarily on improvements in pulmonary function measurements such as FEV1, there is a trend toward greater use of these disease-specific quality-of-life measures to evaluate the benefits of therapy on larger clinical outcomes.
General Approach to Treatment
To be effective, the clinician should address four primary components of management: assess and monitor the condition, avoid or reduce exposure to risk factors, manage stable disease, and treat exacerbations. These components are addressed through a variety of nonpharmacologic and pharmacologic approaches.
Nonpharmacologic Therapy
Patients with COPD should receive education about their disease, treatment plans, and strategies to slow progression and prevent complications.1 Advice and counseling about smoking cessation are essential, if applicable, and should be addressed for patients in all stages of the disease. Because the natural course of the disease leads to respiratory failure, the clinician should address end-of-life decisions and advanced directives prospectively with the patient and family.38
Smoking Cessation
![]() Smoking cessation represents the single most important intervention in preventing the development, as well as the progression, of COPD. A primary component of COPD management is avoidance of or reduced exposure to risk factors. Exposure to environmental tobacco smoke is a major risk factor, and smoking cessation is the most effective strategy to reduce the risk of developing COPD and to slow or stop disease progression. The cost-effectiveness of smoking-cessation interventions compares favorably with interventions made for other major chronic diseases.39 The importance of smoking cessation cannot be overemphasized. Smoking cessation leads to decreased symptomatology and slows the rate of decline of pulmonary function even after significant abnormalities in pulmonary function tests have been detected (FEV1:FVC <60%).31 As confirmed by the Lung Health Study, smoking cessation is the only intervention proven at this time to affect long-term decline in FEV1 and slow the progression of COPD.40 In this 5-year prospective trial, smokers with early COPD were randomly assigned to one of the following three groups: smoking-cessation intervention plus inhaled ipratropium three times a day, smoking-cessation intervention alone, or no intervention. During an 11-year followup, the rate of decline in FEV1 among subjects who continued to smoke was more than twice the rate in sustained quitters. Smokers who underwent smoking-cessation intervention had fewer respiratory symptoms and a smaller annual decline in FEV1 compared with smokers who had no intervention. However, this study also demonstrated the difficulty in achieving and sustaining successful smoking cessation.
Smoking cessation represents the single most important intervention in preventing the development, as well as the progression, of COPD. A primary component of COPD management is avoidance of or reduced exposure to risk factors. Exposure to environmental tobacco smoke is a major risk factor, and smoking cessation is the most effective strategy to reduce the risk of developing COPD and to slow or stop disease progression. The cost-effectiveness of smoking-cessation interventions compares favorably with interventions made for other major chronic diseases.39 The importance of smoking cessation cannot be overemphasized. Smoking cessation leads to decreased symptomatology and slows the rate of decline of pulmonary function even after significant abnormalities in pulmonary function tests have been detected (FEV1:FVC <60%).31 As confirmed by the Lung Health Study, smoking cessation is the only intervention proven at this time to affect long-term decline in FEV1 and slow the progression of COPD.40 In this 5-year prospective trial, smokers with early COPD were randomly assigned to one of the following three groups: smoking-cessation intervention plus inhaled ipratropium three times a day, smoking-cessation intervention alone, or no intervention. During an 11-year followup, the rate of decline in FEV1 among subjects who continued to smoke was more than twice the rate in sustained quitters. Smokers who underwent smoking-cessation intervention had fewer respiratory symptoms and a smaller annual decline in FEV1 compared with smokers who had no intervention. However, this study also demonstrated the difficulty in achieving and sustaining successful smoking cessation.
Tobacco cessation has mortality benefits beyond those related to COPD. A followup analysis of the Lung Health Study data conducted more than 14 years later demonstrated an 18% reduction in all-cause mortality in patients who received the intervention compared with usual care.41 Intervention patients had lower death rates due to coronary artery disease (the leading cause of mortality), cardiovascular diseases, and lung cancer, although all categories did not reach clinical significance.
Every clinician has a responsibility to assist smokers in smoking-cessation efforts. A clinical practice guideline for treating tobacco dependence from the U.S. Public Health Service (PHS) was updated in 2008.42 The major findings and recommendations of that report are summarized in Table 16-9. An earlier report from the Surgeon General in 2004 on the health consequences of smoking broadened the scope of the detrimental effects of cigarette smoking, indicating that “Smoking harms nearly every organ of the body, causing many diseases and reducing the health of smokers in general.”14
All clinicians should take an active role in assisting patients with tobacco dependence in order to reduce the burden on the individual, his or her family, and the healthcare system. It is estimated that over 75% of smokers want to quit and that one-third have made a serious effort. Yet complete and permanent tobacco cessation is difficult.40 Counseling that is provided by clinicians is associated with greater success rates than self-initiated efforts.42
The PHS guidelines recommend that clinicians take a comprehensive approach to smoking-cessation counseling. Advice should be given to smokers even if they have no symptoms of smoking-related disease or if they are receiving care for reasons unrelated to smoking. Clinicians should be persistent in their efforts because relapse is common among smokers owing to the chronic nature of dependence. Brief interventions (3 minutes) of counseling are proven effective. However, it must be recognized that the patient must be ready to stop smoking because there are several stages of decision making. Based on this, a five-step intervention program is proposed (Table 16-10).
TABLE 16-10 Five-Step Strategy for Smoking-Cessation Program (5 A’s)

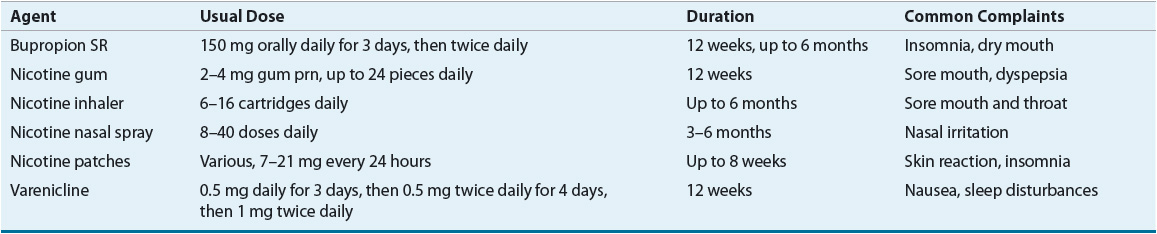
There is strong evidence to support the use of pharmacotherapy to assist in smoking cessation. In fact, it should be offered to most patients as part of a cessation attempt. In general, available therapies will double the effectiveness of a cessation effort. Agents that are considered first line are listed in Table 16-11. The usual duration of therapy is 8 to 12 weeks, although some individuals may require longer courses of treatment. Precautions to consider before using bupropion include a history of seizures or an eating disorder. Nicotine replacement therapies are contraindicated for patients with unstable coronary artery disease, active peptic ulcers, or recent myocardial infarction or stroke. Nicotine patch, bupropion, and the combination of bupropion and the nicotine patch were compared with placebo in a controlled trial.43 The treatment groups that received bupropion had higher rates of smoking cessation than the groups that received placebo or the nicotine patch. The addition of the nicotine patch to bupropion slightly improved the smoking-cessation rate compared with bupropion monotherapy. Recently, a new agent became available to assist in tobacco cessation attempts. Varenicline is a nicotine acetylcholine receptor partial agonist that has shown benefit in tobacco cessation.44 It relieves physical withdrawal symptoms and reduces the rewarding properties of nicotine. Nausea and headache are the most frequent complaints associated with varenicline. Currently, varenicline has not been studied in combination with other tobacco cessation therapies. Second-line agents are less effective or associated with greater side effects; however, they may be useful in selected clinical situations. These therapies include clonidine and nortriptyline, a tricyclic antidepressant.
TABLE 16-11 First-Line Pharmacotherapies for Smoking Cessation
Behavioral modification techniques or other forms of psychotherapy also may be helpful in assisting in smoking cessation. Programs that address the many issues associated with smoking (i.e., learned behaviors, environmental influences, and chemical dependence) using a team approach are more likely to be successful. The role of alternative medicine therapies in smoking cessation is controversial. Hypnosis may aid in improving abstinence rates when added to a smoking-cessation program but appears to give little benefit when used alone. Acupuncture has not been shown to contribute to smoking cessation and is not recommended.3
Other Environmental Triggers
Although cigarette smoke represents the overwhelming majority of risk for developing COPD, exposure to other environmental toxins also confers risks.45,46 Exposures to occupational dusts and fumes have been implicated as a cause of COPD in 19% of smokers and 31% of nonsmokers with COPD in the United States. In the case of known environmental hazards, primary prevention is appropriate. Policies to limit airborne exposures in the workplace and outdoors, as well as education efforts of workers and policy makers, are recommended.
Pulmonary Rehabilitation
Exercise training is beneficial in the treatment of COPD to improve exercise tolerance and to reduce symptoms of dyspnea and fatigue.1 Pulmonary rehabilitation programs are an integral component in the management of COPD and should include exercise training along with smoking cessation, breathing exercises, optimal medical treatment, psychosocial support, and health education. Pulmonary rehabilitation has no direct effect on lung function or gas exchange. Instead, it optimizes other body systems so that the impact of poor lung function is minimized. Exercise training reduces the CNS response to dyspnea, ameliorates anxiety and depression, reduces thoracic hyperinflation, and improves skeletal muscle function.25 High-intensity training (70% maximal workload) is possible even in advanced COPD patients, and the level of intensity improves peripheral muscle and ventilatory function. Studies have demonstrated that pulmonary rehabilitation with exercise three to seven times per week can produce long-term improvement in activities of daily living, quality of life, exercise tolerance, and dyspnea for patients with moderate-to-severe COPD.47 Improvements in dyspnea can be achieved without concomitant improvements in spirometry. While rehabilitation programs vary based on length of program, and exercise frequency and intensity, those with longer length and more frequent sessions have demonstrated the best clinical benefit.
Immunizations
Vaccines can be considered as pharmacologic agents; however, their role is described here in reducing risk factors for COPD exacerbations. Because influenza is a common complication in COPD that can lead to exacerbations and respiratory failure, an annual vaccination with the inactivated intramuscular influenza vaccine is recommended. Immunization against influenza can reduce serious illness and death by 50% in COPD patients.48 Influenza vaccine should be administered in the fall of each year (October and November) during regular medical visits or at vaccination clinics. There are few contraindications to influenza vaccine except for a patient with a serious allergy to eggs. An oral antiinfluenza agent (oseltamivir) can be considered for patients with COPD during an outbreak for patients who have not been immunized; however, this therapy is less effective and causes more side effects.49
The polyvalent pneumococcal vaccine, usually administered one time, is widely recommended for people from 2 to 64 years of age who have chronic lung disease and for all people older than 65 years. Thus, COPD patients at any age are candidates for vaccination. Although evidence for the benefit of the pneumococcal vaccine in COPD is not strong, the argument for continued use is that the current vaccine provides coverage for 85% of pneumococcal strains causing invasive disease and the increasing rate of resistance of pneumococcus to selected antibiotics. Currently, administering the vaccine remains the standard of practice and is recommended by the Centers for Disease Control and Prevention and the American Lung Association. Repeated vaccination with the 23-valent product is not recommended for patients aged 2 to 64 years with chronic lung disease; however, revaccination is recommended for patients over 65 years of age if the first vaccination was more than 5 years earlier and the patient was younger than age 65. The GOLD guidelines recommend pneumococcal vaccine for all COPD patients 65 years and older and for patients less than 65 years only if the FEV1 is less than 40% predicted.50,51 In 2009, the CDC broadened their recommendations for the pneumococcal polysaccharide vaccine to include all persons aged 18 and over who smoke based on a higher risk of pneumococcal infection in these patients.
Long-Term Oxygen Therapy
![]() The use of supplemental oxygen therapy increases survival in COPD patients with chronic hypoxemia. Although long-term oxygen has been used for many years for patients with advanced COPD, it was not until 1980 that data became available documenting its benefits. At that time, the Nocturnal Oxygen Therapy Trial Group published its data comparing nocturnal oxygen therapy (NOT), 12 hours/day, with continuous oxygen therapy (COT), average of 20 hours/day.52 Among patients who were followed for at least 12 months, the results revealed a mortality rate in the NOT group that was nearly double that of the COT group (51% vs. 26%). Statistical estimates of the COT group suggest that COT may have added 3.25 years to a COPD patient’s life. Additional data from the Nocturnal Oxygen Therapy Trial Group revealed that COT patients had fewer (but statistically insignificant) hospitalizations, improved quality of life and neuropsychological function, reduced hematocrit, and decreased pulmonary vascular resistance.52
The use of supplemental oxygen therapy increases survival in COPD patients with chronic hypoxemia. Although long-term oxygen has been used for many years for patients with advanced COPD, it was not until 1980 that data became available documenting its benefits. At that time, the Nocturnal Oxygen Therapy Trial Group published its data comparing nocturnal oxygen therapy (NOT), 12 hours/day, with continuous oxygen therapy (COT), average of 20 hours/day.52 Among patients who were followed for at least 12 months, the results revealed a mortality rate in the NOT group that was nearly double that of the COT group (51% vs. 26%). Statistical estimates of the COT group suggest that COT may have added 3.25 years to a COPD patient’s life. Additional data from the Nocturnal Oxygen Therapy Trial Group revealed that COT patients had fewer (but statistically insignificant) hospitalizations, improved quality of life and neuropsychological function, reduced hematocrit, and decreased pulmonary vascular resistance.52
The decline in mortality with oxygen therapy was further substantiated in 1981 in a study by the British Medical Research Council that compared 15 hours/day of oxygen versus no supplemental oxygen in COPD patients.53 Patients receiving oxygen therapy for at least part of the day had lower rates of mortality than those not receiving oxygen. Long-term oxygen therapy provides even more benefit in terms of survival after at least 5 years of use, and it improves the quality of life of these patients by increasing walking distance and neuropsychological condition and reducing time spent in the hospital.54 Before patients are considered for long-term oxygen therapy, they should be stabilized in the outpatient setting, and pharmacotherapy should be optimized. Once this is accomplished, long-term oxygen therapy should be instituted if either of the following two conditions is observed and documented twice in a 3-week period:
1. A resting PaO2 of less than 55 mm Hg or SaO2 less than 88% with or without hypercapnia
2. A resting PaO2 between 55 and 60 mm Hg or SaO2 less than 88% with evidence of right-sided heart failure, polycythemia, or pulmonary hypertension
The most practical means of administering long-term oxygen is with the nasal cannula, at 1 to 2 L/min, which provides 24% to 28% oxygen. The goal is to raise the PaO2 above 60 mm Hg. Patient education about flow rates and avoidance of flames (i.e., smoking) is of the utmost importance.
There are three different ways to deliver oxygen, including (a) in liquid reservoirs, (b) compressed into a cylinder, and (c) via an oxygen concentrator. Although conventional liquid oxygen and compressed oxygen are quite bulky, smaller, portable tanks are available to permit greater patient mobility. Oxygen concentrator devices separate nitrogen from room air and concentrate oxygen. These are the most convenient and the least expensive method of oxygen delivery. Oxygen-conservation devices are available that allow oxygen to flow only during inspiration, making the supply last longer. These may be particularly useful to prolong the oxygen supply for mobile patients using portable cylinders. However, the devices are bulky and subject to failure.
Adjunctive Therapies
In addition to supplemental oxygen, adjunctive therapies to consider as part of a pulmonary rehabilitation program are psychoeducational care and nutritional support. Psychoeducational care (such as relaxation) has been associated with improvement in the functioning and well-being of adults with COPD.1,3 The role of nutritional support for patients with COPD is controversial. Several studies have shown an association among malnutrition, low body mass index (BMI), and impaired pulmonary status among patients with COPD. However, a meta-analysis suggests that the effect of nutritional support on outcomes in COPD is small and not associated with improved anthropometric measures, lung function, or functional exercise capacity.55
Pharmacologic Therapy
Results from numerous recent clinical trials have improved insight and understanding about the respective roles of various medications used in chronic COPD management; yet, some controversies still exist related to both effectiveness and safety. In contrast to the survival benefit conferred by supplemental oxygen therapy, there is no medication available for the treatment of COPD that has been conclusively shown to modify the progressive decline in lung function or prolong survival.1 There is limited evidence that chronic treatment with long-acting inhaled β-agonists, inhaled corticosteroids (ICs), or the combination can reduce the rate of decline in spirometry in a subset of patients with more severe disease.56 Currently, the primary goal of pharmacotherapy is to control patient symptoms and reduce complications, including the frequency and severity of exacerbations and improving the overall health status and exercise tolerance of the patient.
International guidelines recommend a stepwise approach to the use of pharmacotherapy based on disease severity,1,3 which is determined by the results of spirometry, nature of symptoms, and exacerbation rates. The impact of recurrent exacerbations on accelerating disease progression is increasingly recognized as an important factor to be considered. The primary goals of pharmacotherapy are to control symptoms (including dyspnea), reduce exacerbations, and improve exercise tolerance and health status. Currently, there is inadequate evidence to support the use of more aggressive pharmacotherapy early in the course of disease because of the lack of a disease-modifying benefit. Because of the progressive nature of COPD, pharmacotherapy tends to be chronic and cumulative and step-down approaches in stable patients are not successful. Patients exhibit variable responses to available therapies and the treatment approach should be individualized.
Pharmacotherapy of COPD typically involves the use of inhaled medications, requiring patient knowledge, understanding, and skills using the various inhalation devices. Several delivery devices are available (e.g., metered-dose inhalers [MDIs], dry powder inhalers [DPIs], nebulizers, and ancillary devices such as holding chambers), and the instructions about proper use vary. Comorbidities that are common for patients with COPD, including physical and mental conditions, can have a significant effect on the patient’s ability to use the devices. Periodic and frequent reinforcement and observation by the clinician is required for the patient’s benefit.
![]() Pharmacotherapy focuses on the use of bronchodilators to control symptoms. Bronchodilators relax bronchial smooth muscle, improve lung emptying, reduce thoracic hyperinflation at rest and during exercise, and improve exercise tolerance.1 These effects can be seen in the absence of objective improvements on spirometry. There are several classes of bronchodilators to choose from, and no single class has been proven to provide superior benefit over other available agents. The initial and subsequent choice of medications should be based on the specific clinical situation and patient characteristics. Medications can be used as needed or on a scheduled basis depending on the clinical situation, and additional therapies should be added in a stepwise manner depending on the response and severity of disease. Considerations should be given to individual patient response, tolerabilility, adherence, and economic factors. Recommendations for management of COPD have been proposed based on a combined assessment of airflow limitation, symptoms, and risk of exacerbations, according to the new classification system for disease severity (Fig. 16-4). This schema provides clearer guidance on management compared with previous recommendations, and also allows for the individualization of pharmacotherapy based on patient-specific factors of lung function, symptom frequency and severity, and exacerbation risk.
Pharmacotherapy focuses on the use of bronchodilators to control symptoms. Bronchodilators relax bronchial smooth muscle, improve lung emptying, reduce thoracic hyperinflation at rest and during exercise, and improve exercise tolerance.1 These effects can be seen in the absence of objective improvements on spirometry. There are several classes of bronchodilators to choose from, and no single class has been proven to provide superior benefit over other available agents. The initial and subsequent choice of medications should be based on the specific clinical situation and patient characteristics. Medications can be used as needed or on a scheduled basis depending on the clinical situation, and additional therapies should be added in a stepwise manner depending on the response and severity of disease. Considerations should be given to individual patient response, tolerabilility, adherence, and economic factors. Recommendations for management of COPD have been proposed based on a combined assessment of airflow limitation, symptoms, and risk of exacerbations, according to the new classification system for disease severity (Fig. 16-4). This schema provides clearer guidance on management compared with previous recommendations, and also allows for the individualization of pharmacotherapy based on patient-specific factors of lung function, symptom frequency and severity, and exacerbation risk.
FIGURE 16-4 Pharmacotherapy recommendations based on group classification.




