Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma
Definition
Chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) are considered to represent different manifestations of the same disease in the World Health Organization (WHO) classification (1). The term CLL is used for patients who present primarily with lymphocytosis, and the term “SLL, consistent with CLL” is used when the neoplasm involves lymph nodes without leukemic involvement (1). Most patients with CLL also present with lymphadenopathy, and it is very common for patients with SLL to develop CLL. Chronic lymphocytic leukemia/small lymphocytic leukemia is a neoplasm of small, round, mature-appearing lymphocytes of B-cell lineage with a characteristic (CD5+, CD23+), but not pathognomonic, immunophenotype (1).
Synonyms
Malignant lymphoma, diffuse, well-differentiated lymphocytic (Rappaport); lymphoma, lymphocytic/CLL (Kiel); malignant lymphoma, small lymphocytic, consistent with CLL (Working formulation); CLL [French-American-British (FAB) group].
Epidemiology
In a study using Surveillance, Epidemiology, and End Results (SEER) data in the United States, and spanning the interval from 1992 to 2001, CLL/SLL represented 14.8% of all lymphoid neoplasms and approximately 20% of all B-cell malignancies (2). The overall incidence of CLL/SLL in this study was 5.17 per 100,000 person-years. CLL is the most common form of leukemia in the Western world (3) and represents 90% or more of all chronic lymphoid leukemias. Small lymphocytic lymphoma, without overt leukemia at time of diagnosis, represented 6.7% of all non-Hodgkin lymphomas in one large study (4). Chronic lymphocytic leukemia/small lymphocytic leukemia is more common in whites than in African Americans, and is least common in Asians (2). Chronic lymphocytic leukemia/small lymphocytic leukemia is more common in men than in women. In the WHO classification book, the male-to-female ratio is approximately 2:1, but in the SEER study, the male-to-female ratio was 1.4:1(2).
Chronic lymphocytic leukemia/small lymphocytic leukemia is a neoplasm of late adult life, peaking in the sixth or seventh decade (1,2,3). The median age of affected patients is 65 years (1,4). It is unusual for affected patients to be younger than 40 years of age, and CLL/SLL is truly rare before the age of 20 years (2,3,5). In recent years, with the common use of ancillary techniques such as flow cytometry immunophenotyping, the diagnosis of CLL is being made more frequently in younger patients, who are asymptomatic and present with early-stage disease (5). After 40 years, the incidence increases linearly with age (1,4,6).
The etiology of CLL/SLL in unknown but genetic and environmental factors appear to play a role. The evidence for a genetic component is stronger. In patients with sporadic CLL, the frequency of CLL and other lymphoid malignancies is more common in first-degree relatives than in the general population (7,8). A familial form of CLL also has been described (9). In familial CLL, the phenomenon of anticipation had been described (10), wherein disease onset occurs at younger ages in successive generations. Although sporadic and familial CLL are distinct epidemiologically, they share immunophenotypic and genetic abnormalities (9,10). An increased risk of CLL has been reported in farmers, possibly related to exposure to pesticides, suggesting a role for environmental factors as well (11).
Clinical Syndrome
The clinical course of patients with CLL/SLL is highly variable and correlates, in part, with their presentation as having
either CLL or SLL. Patients who present predominantly with CLL always have lymphocytosis and bone marrow involvement. In one review of CLL, swollen lymph nodes are the most common presenting symptom, and the most common physical findings are lymphadenopathy (87%), splenomegaly (54%), and hepatomegaly (14%) (12). Involved lymph nodes are firm, discrete, mobile, and usually not bulky. Fatigue is common, often attributable to anemia. B-type symptoms, such as fever, weight loss, and night sweats, occur in a subset CLL patients. Approximately 25% of CLL patients are asymptomatic (12).
either CLL or SLL. Patients who present predominantly with CLL always have lymphocytosis and bone marrow involvement. In one review of CLL, swollen lymph nodes are the most common presenting symptom, and the most common physical findings are lymphadenopathy (87%), splenomegaly (54%), and hepatomegaly (14%) (12). Involved lymph nodes are firm, discrete, mobile, and usually not bulky. Fatigue is common, often attributable to anemia. B-type symptoms, such as fever, weight loss, and night sweats, occur in a subset CLL patients. Approximately 25% of CLL patients are asymptomatic (12).
Two clinical staging systems, the Rai and Binet systems, are commonly used to categorize the extent of disease in CLL patients. In the Rai system, five stages are recognized: 0, lymphocytosis in blood and bone marrow and no other disease; I, lymphocytosis with enlarged lymph nodes; II, lymphocytosis with splenomegaly or hepatomegaly (with or without lymphadenopathy); III, lymphocytosis with anemia; and IV, lymphocytosis with thrombocytopenia (13). These stages can be further divided into three risk groups: low (stage 0), intermediate (stages I and II), and high (stages III and IV). The Binet system is based on the number of involved anatomic regions, combined with the levels of hemoglobin and platelets (14). The anatomic regions are the head and neck, axillae, groins, spleen, and liver. In stages A and B, the hemoglobin level is 10 g/dL or more, and the platelet count is 100 ×109/L or higher. In stage A, two or fewer anatomic regions are involved and, in stage B, three or more anatomic regions are involved. In stage C, the hemoglobin level is less than 10 g/dL or the platelet count is less than 100 × 109/L, regardless of the anatomic distribution of disease.
Over 90% of patients who present predominantly with SLL usually have Ann Arbor stage III or IV disease. B-type symptoms occur in one-third of patients, and the serum lactate dehydrogenase level was elevated in 41% of patients in one large study (3). In a significant number of patients with SLL, a leukemic phase may not develop or may occur only after prolonged time intervals (15).
Autoimmune hemolytic anemia, usually Coombs-test positive, occurs in approximately 10% of CLL patients. Patients may have either hypergammaglobulinemia or hypogammaglobulinemia (12). Paraproteins can be detected in the serum of a subset of CLL/SLL patients, with the percentage of patients with paraproteins becoming higher as more sensitive methods of detection are employed. In one study, over half of CLL patients had a low-level monoclonal paraprotein (of any type) in serum or urine detected by immunofixation (16). In our own experience, using standard serum protein electrophoresis and immunofixation, 2.5% of CLL patients have a serum IgM paraprotein (17). Typically, the IgM paraprotein level is low, with a median of 0.4 g/dL, and shares the same immunoglobulin light chain as that expressed by the neoplastic cells. Patients with CLL/SLL are more prone to infections, but this may be attributable to therapy as well as to the disease itself (18). Approximately 5% of patients with CLL/SLL will develop a second, more clinically aggressive lymphoid neoplasm (see later discussion).
Histopathology
Peripheral Blood
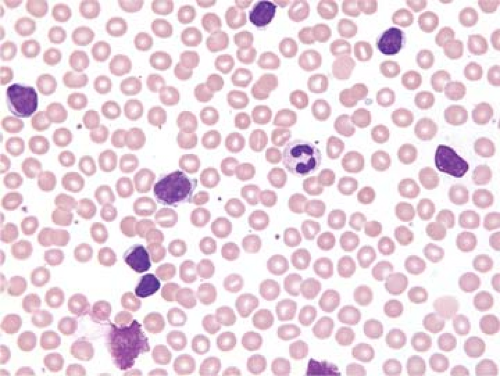
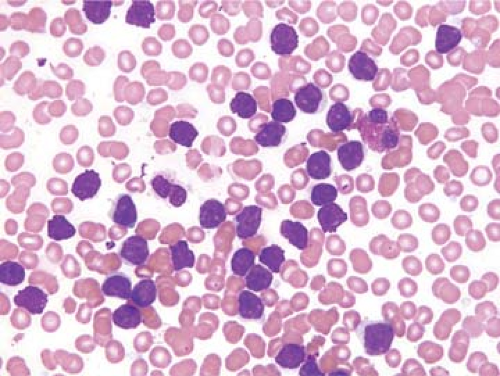
A National Cancer Institute-sponsored working group recommended that the diagnosis of CLL be based on a persistent absolute lymphocytosis of 5 × 109/L or more (19). Others have used a cutoff of 4 × 109/L or more lymphocytes as the minimum criterion for the diagnosis of CLL (15). Lymphocytosis between 4 and 15 × 109/L has been referred to as “low-count” CLL. The lymphocytes in the blood are predominantly small and round with clumped (“soccer ball”) chromatin, inconspicuous or absent nucleoli, and minimal cytoplasm (Fig. 60.1) (20). Mitotic figures are almost never identified in the small lymphocytes but occasional cycling cells, as shown by Ki-67 expression, and dividing cells can be appreciated (21). An occasional prolymphocyte is common in the blood smears of CLL patients, but these usually comprise fewer than 2% to 3% of all lymphocytes. Smudge cells, which represent disrupted cells that occur because CLL cells are fragile, are common (Fig. 60.1). The use of albumin in the preparation of a blood smear influences the cytologic features of the cells, particularly the prolymphocytes, and reduces the number of smudge cells (22).
The FAB group defined three morphologic variants of CLL: typical, CLL/PL, and mixed, not otherwise specified (20). Typical CLL is described as above in the peripheral blood and is similar in bone marrow aspirate smears; 10% or less are prolymphocytes. In CLL/PL, a dimorphic picture of small lymphocytes and prolymphocytes is present, and prolymphocytes range from more than 10% to less than 55%. In mixed type CLL not otherwise specified, a spectrum of small and large lymphocytes is present with prolymphocytes numbering less than 10%. In the literature, the CLL/PL group appears to be more refractory to treatment, with a poorer prognosis (20).
As reviewed by Matutes and Polliak (23), others have subdivided CLL into typical and atypical groups. Atypical CLL is defined by (a) the presence of increased (>10%) prolymphocytes, and (b) the presence of increased (>15%) lymphoplasmacytic or cleaved cells. Cases of atypical CLL are frequently associated with more advanced stage and/or poorer survival, an atypical immunophenotype, and the presence of cytogenetic abnormalities such as trisomy 12 and p53 gene mutations.
Bone Marrow
The lymphocytes in bone marrow aspirate smears resemble those seen in the peripheral blood (Fig. 60.2). In aspirate clot and biopsy sections, three patterns of bone marrow involvement are described in CLL/SLL: nodular, interstitial, and diffuse (Fig. 60.3). These patterns correlate with overall tumor burden and patient prognosis (24). Although seemingly clear at first glance, the use of these terms to describe CLL patterns
is not entirely reproducible from observer to observer, so our definitions for these patterns follow. The nodular pattern is composed of non-paratrabecular aggregates of small lymphocytes that can be vaguely nodular or more circumscribed (Fig. 60.3A). The interstitial pattern is characterized by more extensive infiltration by small lymphocytes throughout the bone marrow, with preservation of the fat cells (Fig. 60.3B). In the diffuse pattern, medullary fat cells are partially to extensively replaced by sheets of small lymphocytes (Fig. 60.3C). Transitional or combination patterns also are commonly observed. Proliferation centers can be seen in the bone marrow but, in our experience, usually only in cases with relatively extensive involvement (Fig. 60.3C).
is not entirely reproducible from observer to observer, so our definitions for these patterns follow. The nodular pattern is composed of non-paratrabecular aggregates of small lymphocytes that can be vaguely nodular or more circumscribed (Fig. 60.3A). The interstitial pattern is characterized by more extensive infiltration by small lymphocytes throughout the bone marrow, with preservation of the fat cells (Fig. 60.3B). In the diffuse pattern, medullary fat cells are partially to extensively replaced by sheets of small lymphocytes (Fig. 60.3C). Transitional or combination patterns also are commonly observed. Proliferation centers can be seen in the bone marrow but, in our experience, usually only in cases with relatively extensive involvement (Fig. 60.3C).
Lymph Node
The lymph node architecture is totally or subtotally replaced by lymphoma (25,26). The neoplasm usually obliterates the follicles and sinuses and spreads through the capsule into the surrounding adipose tissue (Fig. 60.4). The pattern of the neoplasm is diffuse, but pale, poorly defined nodular areas, known as proliferation centers, pseudofollicular growth centers, or pseudofollicles (henceforth referred to as pseudofollicles) are identified within the overall diffuse pattern (Figs. 60.5) and 60.6) (25,26). The pseudofollicles are best appreciated at low-power magnification; decreasing the light intensity of the microscope, by enhancing contrast, increases the visibility of the pseudofollicles, which appear pale relative to the remainder of the neoplasm (Figs. 60.5A and 60.6A). The pseudofollicles appear pale because the distances between the cell nuclei in the pseudofollicles are greater than between the more densely packed small lymphocytes in the remainder of the neoplasm (Fig. 60.5B). If a lymph node biopsy specimen is of adequate
size, and fixed and processed well, pseudofollicles can be found in most cases of CLL/SLL.
size, and fixed and processed well, pseudofollicles can be found in most cases of CLL/SLL.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree