Drugs with acetylcholine-like effects (cholinomimetics) consist of 2 major subgroups on the basis of their mode of action (ie, whether they act directly at the acetylcholine receptor or indirectly through inhibition of cholinesterase). Drugs in the direct-acting subgroup are further subdivided on the basis of their spectrum of action (ie, whether they act on muscarinic or nicotinic cholinoceptors).
Acetylcholine may be considered the prototype that acts directly at both muscarinic and nicotinic receptors. Neostigmine is a prototype for the indirect-acting cholinesterase inhibitors.
High-Yield Terms to Learn
Choline ester A cholinomimetic drug consisting of choline (an alcohol) esterified with an acidic substance, (eg, acetic or carbamic acid); usually poorly lipid-soluble Cholinergic crisis The clinical condition of excessive activation of cholinoceptors; it may include skeletal muscle weakness as well as parasympathetic signs Cholinomimetic alkaloid A drug with weakly basic properties (usually of plant origin) whose effects resemble those of acetylcholine; usually lipid-soluble Cyclospasm Marked contraction of the ciliary muscle; maximum accommodation for close vision Direct-acting cholinomimetic A drug that binds and activates cholinoceptors; the effects mimic those of acetylcholine Endothelium-derived relaxing factor (EDRF) A potent vasodilator substance, largely nitric oxide (NO), that is released from vascular endothelial cells Indirect-acting cholinomimetic A drug that amplifies the effects of endogenous acetylcholine by inhibiting acetylcholinesterase Muscarinic agonist A cholinomimetic drug that binds muscarinic receptors and has primarily muscarine-like actions Myasthenic crisis In patients with myasthenia, an acute worsening of symptoms; usually relieved by increasing cholinomimetic treatment Nicotinic agonist A cholinomimetic drug that binds nicotinic receptors and has primarily nicotine-like actions Organophosphate An ester of phosphoric acid and an alcohol that inhibits cholinesterase Organophosphate aging A process whereby the organophosphate, after binding to cholinesterase, is chemically modified and becomes more firmly bound to the enzyme Parasympathomimetic A drug whose effects resemble those of stimulating the parasympathetic nerves
Direct-Acting Cholinomimetic Agonists
This class comprises a group of choline esters (acetylcholine, methacholine, carbachol, and bethanechol) and a second group of naturally occurring alkaloids (muscarine, pilocarpine, nicotine, lobeline). Newer drugs are occasionally introduced for special applications. The members differ in their spectrum of action (amount of muscarinic versus nicotinic stimulation) and in their pharmacokinetics (Table 7-1). Both factors influence their clinical use.
TABLE 7-1 Some cholinomimetics: spectrum of action and pharmacokinetics.
Drug Spectrum of Actiona
Pharmacokinetic Features Direct-acting Acetylcholine B Rapidly hydrolyzed by cholinesterase (ChE); duration of action 5-30 s; poor lipid solubility Bethanechol M Resistant to ChE; orally active, poor lipid solubility; duration of action 30 min to 2 h Carbachol B Like bethanechol Pilocarpine M Not an ester, good lipid solubility; duration of action 30 min to 2 h Nicotine N Like pilocarpine; duration of action 1-6 h; high lipid solubility Varenicline N Partial agonist at N receptors, high lipid solubility; duration 12-24 h Indirect-acting Edrophonium B Alcohol, quaternary amine, poor lipid solubility, not orally active; duration of action 5-15 min Neostigmine B Carbamate, quaternary amine, poor lipid solubility, orally active; duration of action 30 min to 2 h or more Physostigmine B Carbamate, tertiary amine, good lipid solubility, orally active; duration of action 30 min to 2 h Pyridostigmine B Carbamate-like neostigmine, but longer duration of action (4-8 h) Echothiophate B Organophosphate, moderate lipid solubility; duration of action 2-7 days Parathion B Organophosphate, high lipid solubility; duration of action 7-30 days
aB, both; M, muscarinic; N, nicotinic.
Classification
Muscarinic agonists are parasympathomimetic; that is, they mimic the actions of parasympathetic nerve stimulation in addition to other effects. Five subgroups of muscarinic receptors have been identified (Table 7-2), but the muscarinic agonists available for clinical use activate them nonselectively. Nicotinic agonists are classified on the basis of whether ganglionic or neuromuscular stimulation predominates; however, agonist selectivity is limited. On the other hand, relatively selective antagonists are available for the two nicotinic receptor types (Chapter 8).
TABLE 7-2 Cholinoceptor types and their postreceptor mechanisms.
Receptor Type G Protein Postreceptor Mechanisms M1
Gq
 IP3, DAG cascade
IP3, DAG cascade
M2
Gi
 cAMP synthesis M3
cAMP synthesis M3
Gq
 IP3, DAG cascade
IP3, DAG cascade
M4
Gi
 cAMP synthesis M5
cAMP synthesis M5
Gq
 IP3, DAG cascade
IP3, DAG cascade
NM
None Na+/K+ depolarizing current
NN
None Na+/K+ depolarizing current
cAMP, cyclic adenosine monophosphate; DAG, diacylglycerol; IP3 inositol-1,4,5-trisphosphate
Molecular Mechanisms of Action
Muscarinic Mechanism
Several molecular mechanisms of muscarinic action have been defined (Table 7-2). One involves Gq-protein coupling of M1 and M3 muscarinic receptors to phospholipase C, a membrane-bound enzyme, leading to the release of the second messengers, diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (IP 3). DAG modulates the action of protein kinase C, an enzyme important in secretion, whereas IP3 evokes the release of calcium from intracellular storage sites, which results in contraction in smooth muscle. A second mechanism couples M2 muscarinic receptors to adenylyl cyclase through the inhibitory Gi-coupling protein. A third mechanism couples the same M2 receptors via the 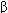
 subunit of the G protein to potassium channels in the heart and elsewhere; muscarinic agonists facilitate opening of these channels. M4 and M5 receptors may be important in the central nervous system (CNS) but have not been shown to play major roles in peripheral organs.
subunit of the G protein to potassium channels in the heart and elsewhere; muscarinic agonists facilitate opening of these channels. M4 and M5 receptors may be important in the central nervous system (CNS) but have not been shown to play major roles in peripheral organs.
Nicotinic Mechanism
The mechanism of nicotinic action has been clearly defined. The nicotinic acetylcholine ACh receptor is located on a channel protein that is selective for sodium and potassium. When the receptor is activated, the channel opens and depolarization of the cell occurs as a direct result of the influx of sodium, causing an excitatory postsynaptic potential (EPSP). If large enough, the EPSP evokes a propagated action potential in the surrounding membrane. The nicotinic receptors on sympathetic and parasympathetic ganglion cells (NN, also denoted NG) differ slightly from those on neuromuscular end plates (NM).
Tissue and Organ Effects
The tissue and organ system effects are summarized in Table 7-3. Note that vasodilation (and decreased blood pressure) is not a parasympathomimetic response (ie, it is not evoked by parasympathetic nerve discharge, even though directly acting cholinomimetics cause vasodilation). This action results from the release of endothelium-derived relaxing factor (EDRF; nitric oxide and possibly other substances) in the vessels, mediated by uninnervated
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


