42
Chemotherapy of Tuberculosis, Mycobacterium Avium Complex Disease, and Leprosy
This chapter will be most useful after having a basic understanding of the material in Chapter 56, Chemotherapy of Tuberculosis, Mycobacterium Avium Complex Disease, and Leprosy in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. In addition to the material presented here, the 12th Edition contains:
• Table 56-1 Pathogenic Mycobacterial Rapid and Slow Growers (Runyon Classification)
• Table 56-2 Population Pharmacokinetic Parameter Estimates for Antimycobacteial Drugs in Adult Patients
• Table 56-3 Pharmacokinetic Parameters of Rifampin, Rifabutin, and Rifapentine
• Table 56-5 Drugs Used in the Treatment of Mycobacteria Other Than for Tuberculosis, Leprosy, or MAC (Mycobacterium Avium Complex)
• Figure 56-4 Multimodal distribution of isoniazide (INH) clearance due to NAT2 polymorphisms
DRUGS INCLUDED IN THIS CHAPTER
• Aminoglycosides (streptomycin, amikacin, kanamycin) (See Chapter 40)
• Capreomycin (CAPASTAT)
• Clofazimine (LAMPRENE)
• Cycloserine (SEROMYCIN)
• Dapsone
• Ethambutol (MYAMBUTOL)
• Ethionamide (TRECATOR)
• Fluoroquinolones (ofloxacin, ciprofloxacin, moxifloxacin) (See Chapter 38)
• Isoniazid (NYDRAZID)
• PA-824
• Pyrazinamide
• Rifamycins: Rifampin (RIFADIN, RIMACTANE, others), Rifapentine (PRIFTIN), Rifabutin (MYCOBUTIN)
• TMC-207
LEARNING OBJECTIVES
 Understand the rationale for combination drug therapy in the treatment of tuberculosis (TB).
Understand the rationale for combination drug therapy in the treatment of tuberculosis (TB).
 Know the mechanisms of action and resistance for drugs used to treat TB and leprosy.
Know the mechanisms of action and resistance for drugs used to treat TB and leprosy.
 Describe the adverse effects and drug interactions commonly associated with anti-TB drugs.
Describe the adverse effects and drug interactions commonly associated with anti-TB drugs.
 Know the principles of anti-TB chemotherapy.
Know the principles of anti-TB chemotherapy.
 Understand the principles of therapy of Mycobacterium Avium Complex (MAC) disease.
Understand the principles of therapy of Mycobacterium Avium Complex (MAC) disease.
 Understand the principles of antileprosy therapy.
Understand the principles of antileprosy therapy.
MECHANISMS OF ACTION AND RESISTANCE OF DRUGS USED TO TREAT TUBERCULOSIS AND LEPROSY
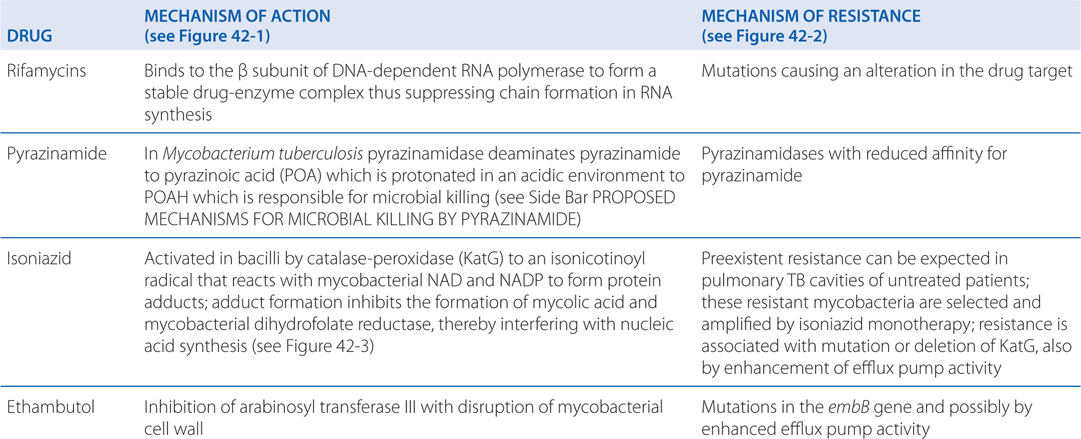
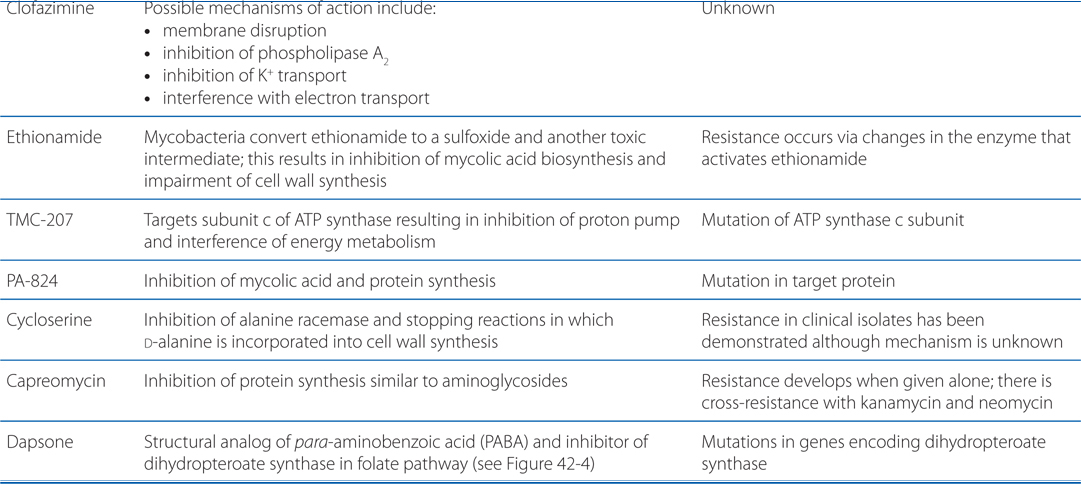
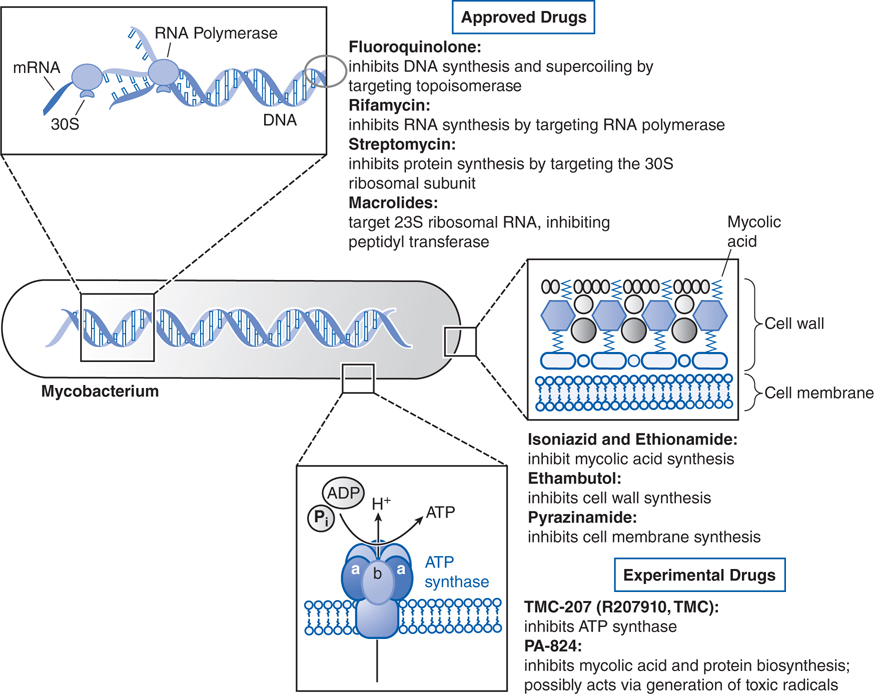
FIGURE 42-1 Mechanisms of action of established and experimental drugs used for the chemotherapy of mycobacterial infections. Shown at the top are the sites of action of approved drugs for the chemotherapy of mycobacterial diseases. Rifamycin is used as a generic term for several drugs, of which rifampin is used most frequently. Also included are 2 experimental drugs now under investigation: TMC-207 and PA-824. Clofazimine, whose mode of action is not understood, is omitted.
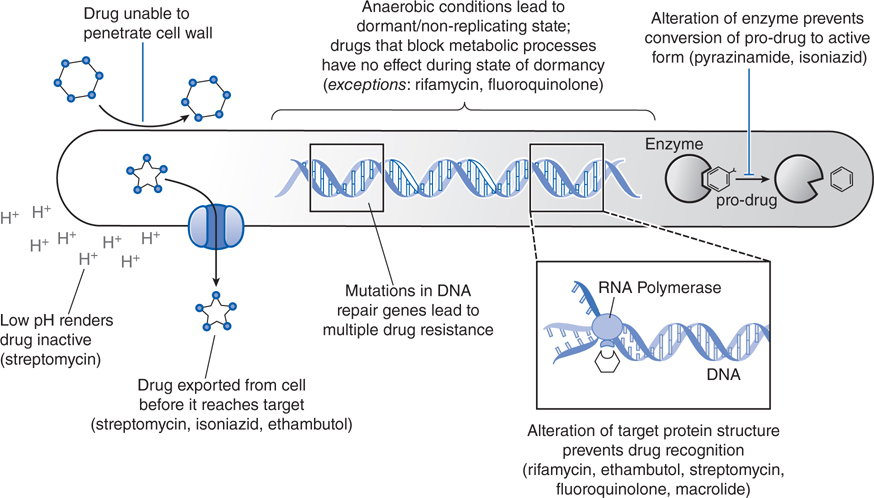
FIGURE 42-2 Mechanisms of resistance of Mycobacteria to different chemotherapeutic drugs. Shown are the various mechanisms by which mycobacteria resist antibacterial effects of the currently approved chemotherapeutic agents.
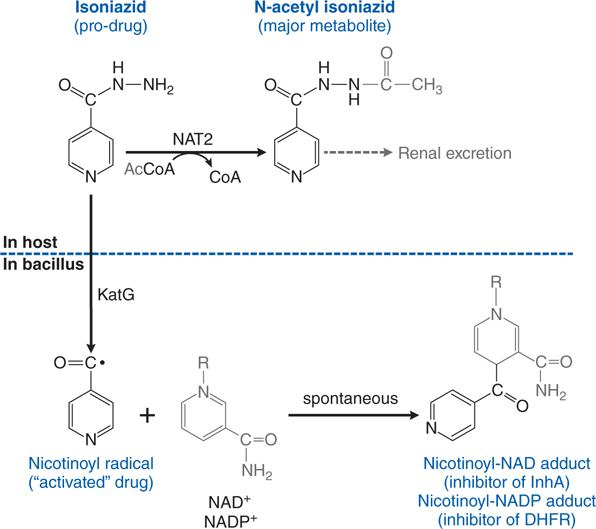
FIGURE 42-3 Metabolism and activation of isoniazid. The prodrug isoniazid is metabolized in humans by NAT2 isoforms to its principal metabolite, N-acetyl isoniazid, which is excreted by the kidney. Isoniazid diffuses into mycoplasma where it is “activated” by KatG (oxidase/peroxidase) to the nicotinoyl radical, which reacts spontaneously with NAD+ or NADP+ to produce adducts that inhibit important enzymes in cell wall and nucleic acid synthesis. DHFR, dihydrofolate reductase.
• Inhibition of fatty acid synthesis type I leading to interference with mycolic acid synthesis
• Reduction of intracellular pH
• Disruption of membrane transport by protonated pyrazinoic acid (POAH)
CRITERION FOR PROPHYLACTIC ANTITUBERCULOSIS THERAPY
Mantoux reaction is more than or equal to 5 mm and those who meet one of following criteria:
• Recently exposed to TB
• HIV co-infection
• Fibrotic changes on chest radiograms
• Immunosuppressed
Mantoux reaction is more than or equal to 10 mm and those who meet one of the following criteria:
• Recent (≤5 years) immigrants from area of high TB prevalence
• Children younger than 4 years
• Children exposed to adults with TB
• Intravenous (IV) drug users
• Residents and employees in high-risk settings
Anyone with a Mantoux reaction more than 15 mm
DEFINITIVE ANTITUBERCULOSIS THERAPY
All active TB cases should be confirmed by culture with antimicrobial susceptibility determined.
For the first 2 months:
• Isoniazid (5 mg/kg, maximum 300 mg/d), pyridoxine (10 to 50 mg/d)
• Rifampin (10 mg/kg, maximum 600 mg/d)
• Pyrazinamide (15 to 30 mg/kg, maximum 2 g/d)
Follow-up therapy, 2 or 3 times per week, for next 4 months:
• Isoniazid (15 mg/kg), pyridoxine (10 to 50 mg/d)
• Rifampin (10 mg/kg)
On a recent trip to India, a 45-year-old man contracted tuberculosis that was confirmed by culture. Antimicrobial susceptibility was performed and the patient was started on isoniazid, rifampin, and pyrazinamide as definitive therapy for tuberculosis.
a. Why did this patient receive three drugs?
Only combination anti-TB therapy is currently recommended for treatment of TB. When anti-TB drug monotherapy was administered to TB patients, resistance emergence terminated the effectiveness of these drugs. The mutation rates to first-line TB drugs are between 10–7 and 10–10 so that the likelihood of resistance is high to any single anti-TB drug in patients with cavitary TB who have ~109 CFU (colony forming units) of bacilli in a 3-cm pulmonary lesion. However, the likelihood that bacilli would develop mutations to 2 or more different drugs is the product of 2 mutation rates which makes the probability of resistance emergence to more than 2 drugs acceptably small. Multidrug therapy has also led to a reduction in length of therapy.
b. What is the mechanism of action of rifampin?
Rifampin enters bacilli in a concentration-dependent manner where it binds to the β subunit of DNA-dependent RNA polymerase to form a stable drug-enzyme complex. Drug binding suppresses chain formation in RNA synthesis (see Figure 42-1).
c. How should rifampin therapy be optimized?
Rifampin’s bactericidal activity is best optimized by a regimen to achieve a high AUC/MIC (area under the curve/minimal inhibitory concentration) ratio, but resistance suppression and rifampin’s enduring postantibiotic effect are best optimized by high CPmax/MIC therapy (see Chapter 34). Therefore, the duration of time that the rifampin concentration persists above the MIC is of less importance than reaching a high CPmax. The t½ of rifampin is less of an issue in optimizing therapy, and if patients could tolerate it, higher doses would lead to higher bactericidal activities while suppressing resistance.
d. For what clinical diseases, other than TB, might rifampin be useful?
Rifampin is also useful for the prophylaxis of meningococcal disease and Haemophilus influenza meningitis. Combined with a β-lactam antibiotic or vancomycin, rifampin may be useful for therapy in selected cases of staphylococcal endocarditis or osteomyelitis, especially those caused by staphylococci “tolerant” to penicillin. Rifampin may also be used for the eradication of staphylococcal nasal carrier state.
e. What adverse effects should this patient be warned of with the use of rifampin?
Rifampin is generally well tolerated, but patients should be warned that it will cause an orange-tan discoloration of skin, urine, feces, saliva, tears, and contact lenses.
A 35-year-old woman hospital employee has a routine tuberculin skin test reaction of 18 mm. She is started on prophylactic isoniazid therapy of 300 mg daily. She is also started on pyridoxine (vitamin B6).
a. What is the mechanism of action of isoniazid?
Isoniazid is activated within the bacillus to an isonicotinoyl radical by KatG, a multifunctional catalase-peroxidase (see Figure 42-3). This radical interacts with mycobacterial NAD and NAPD to form adducts that inhibit the activities of enzymes responsible for the synthesis of mycolic acid, an essential component of the mycobacterial cell wall. The adducts also inhibit mycobacterial dihydrofolate reductase and interfere with nucleic acid synthesis.
b. How is isoniazid metabolized?
Isoniazid is metabolized by hepatic arylamine N-acetyltransferase type 2 (see Figure 42-3). Isoniazid clearance in patients can be classified into 2 phenotypic groups: slow and fast acetylators as seen in Figure 56-4 of Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. Recently the phenotypic groups have been expanded to fast, intermediate, and slow acetylators. The number of NAT2*4 alleles accounts for 88% of the variability of isoniazid clearance. Slow acetylators may be a greater risk for adverse effects from isoniazid, sulfonamides, and procainamide. Fast acetylators may have diminished responses to standard doses of these agents but a greater risk from bioactivation by NAT2 of arylamine/hydrazine carcinogens.
FIGURE 42-4 Effects of antimicrobials on folate metabolism and deoxynucleotide synthesis.
In the patient in Case 42-2:
a. What are the potential side effects of isoniazid therapy in this otherwise healthy woman?
Isoniazid is converted to acetylisoniazid which can be converted to acetylhydrazine and hepatotoxic metabolites by CYP2E1. Rapid acetylators will form diacetylhydrazine which is nontoxic, while slow acetylators or CYP2E1 induction will lead to more hepatotoxic metabolites. Rifampin, a potent inducer of CYP2E1, potentiates isoniazid hepatotoxicity.
Isoniazid also can cause a peripheral neuritis (see answer to Case 42-3c below).
b. What drug interactions should she be warned of?
Isoniazid is a potent inhibitor of CYP2C19, CYP3A, and a weak inhibitor of CYP2D6. Isoniazide induces CYP2E1. Drugs metabolized by these enzymes will potentially be affected. Table 42-1 lists the potential drug interactions that might occur with isoniazid and their adverse effects.
TABLE 42-1 Isoniazid-Drug Interactions via Inhibition and Induction of CYPs

c. Why is she also given a vitamin?
As mentioned above in the answer to question a of this case, isoniazid causes a peripheral neuritis (most commonly paresthesias of the feet and hands). This neuropathy is more frequent in slow acetylators and in individuals with diabetes mellitus, poor nutrition, or anemia. The prophylactic administration of pyridoxine (vitamin B6) prevents the development of peripheral neuritis even when therapy lasts as long as 2 years.
A 25-year-old medical resident has developed active TB after being exposed in the hospital. It is known that the patient she was exposed to had a strain of M. tuberculosis that was resistant to isoniazid. She is started on rifampin, pyrazinamide, and ethambutol.
a. What is the mechanism of action of ethambutol?
Ethambutol inhibits arabinosyl transferase III which disrupts the assembly of the mycobacterial cell wall.
b. If this patient’s M. tuberculosis is resistant to isoniazid, might it also be resistant to ethambutol?
Mycobacterial resistance to ethambutol develops via mutations in the embB gene. However, enhanced efflux pump activity may induce resistance to both isoniazid and ethambutol. Thus, resistance to isoniazid does not necessarily predict resistance to ethambutol.
c. What serious side effect of ethambutol should this patient be warned about?
About 1% of patients receiving ethambutol experience diminished visual acuity. Approximately 15% of patients receiving a dose of ethambutol of 50 mg/kg/d will develop optic neuritis resulting in decreased visual acuity and an inability to distinguish red from green. The incidence of this reaction is proportional to the dose of ethambutol with approximately 1% of patients who receive a dose of 15 mg/kg/d experiencing this effect.
A 36-year-old man in Central Africa has developed leprosy. He is being treated with rifampin, clofazimine, and dapsone.
a. Why 3 drugs?
The reasons for using combination therapy in the treatment of leprosy include reduction in the development of resistance, the need for adequate therapy when primary resistance already exists, and reduction in the duration of therapy. Rifampin is the most bactericidal drug in the regimen. Clofazimine is only bacteriostatic against Myocobacterium leprae; however, it also has anti-inflammatory effects.
b. What is the mechanism of action of dapsone?
Dapsone is a competitive inhibitor of dihydropteroate synthase in the folate pathway (see Figure 42-4). Dapsone also has anti-inflammatory effects by inhibiting tissue damage caused by neutrophils.
c. What enzyme deficiency should this patient be tested for before starting dapsone therapy?
Dapsone, an oxidant, causes severe hemolysis in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency. Thus, G6PD deficiency testing should be performed prior to the use of dapsone wherever possible.
TREATMENT OF DRUG-RESISTANT TUBERCULOSIS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree